Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-THE SOLID STATE-Numerical Problems
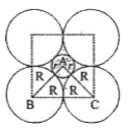
- If the radius of the octahedral void is r and the radius of the atoms...
Text Solution
|
- A compound is formed by two elements X and Y. Atoms of the element Y (...
Text Solution
|
- Atoms of element B form hep lattice and those of the elements A occupy...
Text Solution
|
- In a face centered cubic arrangement of A and B atoms whose A atoms ar...
Text Solution
|
- A unit cell consists of a cube in which there are anions Y at each cor...
Text Solution
|
- If three elements P, Q and R crystalline in a cubic solid lattice with...
Text Solution
|
- A cube solid is made up of two elements P and Q. Atoms Q are present a...
Text Solution
|
- Gold (atomic radius=0.144nm) crystallises in a face-centred unit cell ...
Text Solution
|
- Aluminium crystallises in a cubic close packed structure. Its metallic...
Text Solution
|
- Aluminium crystallises in a cubic close packed structure. Its metallic...
Text Solution
|
- Silver crystallisaes in fcc lattice. If the edge length of th cell is ...
Text Solution
|
- Copper crystallizes into a fcc lattice with edge length 3.61xx10^(-8) ...
Text Solution
|
- An element with molar mass 2.7xx10^(-2) kg "mol"^(-1) forms a cubic un...
Text Solution
|
- Silver crystallisaes in fcc lattice. If the edge length of th cell is ...
Text Solution
|
- Silver forms cpp lattice and X-ray studies of its crystals show that t...
Text Solution
|
- Sodium has a b.c.c. structure with nearest neighbour distance 365.9 pm...
Text Solution
|
- Analysis shows that nickel oxide has the formula Ni(0.98)O. What fract...
Text Solution
|
- When NaCl is dopped with 10^(-5) " mole % of" SrCl(2), what is the no....
Text Solution
|
- The composition of a simple of Wustrie is Fe(0.03)O. What is the perce...
Text Solution
|
- A metal having atomic mass 50 g "mol"^(-1) has a body centred cubic cr...
Text Solution
|
- An element has a body-centred cubic (bbc) structure with a cell edge o...
Text Solution
|