Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
BETTER CHOICE PUBLICATION|Exercise ELECTROLYTIC CELLS AND ELECTROLYSIS|10 VideosELECTROCHEMISTRY
BETTER CHOICE PUBLICATION|Exercise FUEL CELLS|8 VideosELECTROCHEMISTRY
BETTER CHOICE PUBLICATION|Exercise NERNST EQUATION|4 VideosCO-ORDINATION COMPOUNDS
BETTER CHOICE PUBLICATION|Exercise QUESTION FROM PREVIOUS BOARD EXAMINATION|59 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
BETTER CHOICE PUBLICATION|Exercise Question Bank (6.6 OXIDATION-REDUCTION)|15 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ELECTROCHEMISTRY-CONDUCTANCE OF ELECTROLYTIC SOLUTIONS
- Define conductance and conductivity?
Text Solution
|
- Define Specific conductance.
Text Solution
|
- Define conductivity and molar conductivity for the solution of an elec...
Text Solution
|
- What is electrical conductivity? Name the metals which have the highes...
Text Solution
|
- What is electrical conductivity? Name the metals which have the highes...
Text Solution
|
- What is electrical conductivity in ionic solids due to ?
Text Solution
|
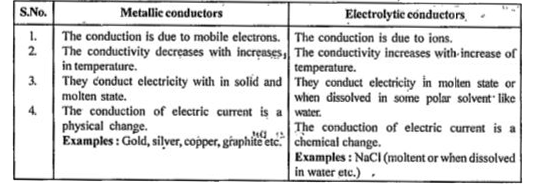
- What are metallic conductors ?
Text Solution
|
- What are electrolyte conductors ?
Text Solution
|
- What is weak electrolyte?
Text Solution
|
- What are metallic conductors ?
Text Solution
|
- How does electrolytic conductance vary with concentration of weak elec...
Text Solution
|
- Write two difference between strong and weak electrolytes.
Text Solution
|
- How does electrolytic conductance vary with concentration of weak elec...
Text Solution
|
- How does the electrolytic conductance vary with temperature ?
Text Solution
|
- Kohlrausch law can be used to find the molar conductivity of a weak el...
Text Solution
|
- State and explain Kohlrausch's law. How would you determine the molar ...
Text Solution
|
- Assume that you know the molar conductances of NH(4)Cl, NaOH and NaCl ...
Text Solution
|
- Assume that you know the molar conductances of HCl, NaCl and CH(3)COO ...
Text Solution
|
- Assume that you know the molar conductances of AgNO(3), KCl and KNO(3)...
Text Solution
|
- Suggest a way to determine the A^@m value of water.
Text Solution
|