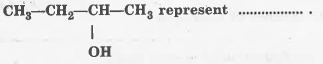Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
BETTER CHOICE PUBLICATION|Exercise FUEL CELLS|8 VideosCO-ORDINATION COMPOUNDS
BETTER CHOICE PUBLICATION|Exercise QUESTION FROM PREVIOUS BOARD EXAMINATION|59 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
BETTER CHOICE PUBLICATION|Exercise Question Bank (6.6 OXIDATION-REDUCTION)|15 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ELECTROCHEMISTRY-Numerical Problems
- Complete the reaction :
Text Solution
|
- Write Nernst equation and calculate the e.m.f of the following cell at...
Text Solution
|
- Fill in the blanks:
Text Solution
|
- Calculate the standard Gibbs energy for the cell : Zn(s)+Zn^(2+)(aq)...
Text Solution
|
- Calculate the standard Gibbs energy for the cell : Zn(s)|Zn^(2+)(aq)...
Text Solution
|
- Calculate the standard Gibbs energy for the cell : Zn(s)|Zn^(2+)(aq)...
Text Solution
|
- The lamda^(@) values of KNO(3) and LiNO(3) are 145.0 and 110.1 S cm^(2...
Text Solution
|
- Calculate the e.m.f. of the following cell at 298 K. Mg((s))|Mg^(2+)...
Text Solution
|
- The lamda^(@) values of KCl and KNO(3) are 149.9 and 144.9 S cm^(2)mol...
Text Solution
|
- Calculate the e.m.f. of the following cell at 298 K, Mg|Mg^(2+)(0.13...
Text Solution
|
- The lamda^(@) values of NaCl and NanO(3) are 126.5 and 121.6S" "cm^(2)...
Text Solution
|
- The calculate the e.mf. Of the following cell at 298: Fe|Fe^(2+)(0.1...
Text Solution
|
- Write the Nernst equation and calculate e.m.f of following cell at 298...
Text Solution
|
- Write Nernst equation and calculate the e.m.f. of the following cell a...
Text Solution
|
- Write Nernst equation and calculate e.m.f. of the cell at 298 k. Mg(...
Text Solution
|
- Write Nernst equation and calculate the e.m.f of the following cell at...
Text Solution
|
- Write Nernst equation and calculate the e.m.f. of the following cell a...
Text Solution
|
- Calculate the e.m.f. of the cell at 25^(@)C Cr|Cr^(3+)(0.1M)||Fe^(2+)(...
Text Solution
|
- What is the amount of electricity required to deposit one mole of alum...
Text Solution
|
- A solution of Ni(NO3)2 is electrolysed between platinum electrodes usi...
Text Solution
|