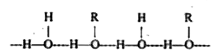Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS , PHENOLS AND ETHERS
BETTER CHOICE PUBLICATION|Exercise 11. 4 . 3 . CHEMICAL PROPERTIES |61 VideosALCOHOLS , PHENOLS AND ETHERS
BETTER CHOICE PUBLICATION|Exercise 11. 5 SOME COMMERCIALLY IMPORTANT ALCOHOLS |9 VideosALCOHOLS , PHENOLS AND ETHERS
BETTER CHOICE PUBLICATION|Exercise QUESTION BANK 11 . 1 CLASSIFICATION |24 VideosALTERNATING CURRENTS
BETTER CHOICE PUBLICATION|Exercise MOST EXPECTED QUESTIONS|11 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ALCOHOLS , PHENOLS AND ETHERS -11 . 4. 2 PHYSICAL PROPERTIES
- Explain why propanol has higher boiling than of the hydrocarbon b...
Text Solution
|
- Why do alcohols have higher boiling points than halo-alkanes of the sa...
Text Solution
|
- Why do alcohols have higher boiling points than haloalkanes ?
Text Solution
|
- Why are alcohols comparatively more soluble in water than the correspo...
Text Solution
|
- Alcohols are soluble in water while alkyl halides arenot, although bot...
Text Solution
|
- Why are alcohols soluble in water ?
Text Solution
|
- Why are alcohols soluble in water ?
Text Solution
|
- Why solubility of alcohols in water decreases with increase in molecul...
Text Solution
|
- Why boiling point of phenols are higher than aromatic hydrocarb...
Text Solution
|
- Why has phenol, higher boiling point than toluene ?
Text Solution
|