Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS , PHENOLS AND ETHERS
BETTER CHOICE PUBLICATION|Exercise 11. 5 SOME COMMERCIALLY IMPORTANT ALCOHOLS |9 VideosALCOHOLS , PHENOLS AND ETHERS
BETTER CHOICE PUBLICATION|Exercise 11.6 . ETHERS |8 VideosALCOHOLS , PHENOLS AND ETHERS
BETTER CHOICE PUBLICATION|Exercise 11 . 4. 2 PHYSICAL PROPERTIES |10 VideosALTERNATING CURRENTS
BETTER CHOICE PUBLICATION|Exercise MOST EXPECTED QUESTIONS|11 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ALCOHOLS , PHENOLS AND ETHERS -11. 4 . 3 . CHEMICAL PROPERTIES
- Orthonitrophenol and paranitrophenol are more acidic than phenols. Giv...
Text Solution
|
- Alcohols are easily protonated in comparison to phenols.
Text Solution
|
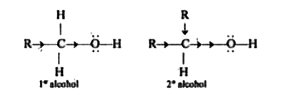
- Why primary alcohols are more acidic than secondary alcohols?
Text Solution
|
- Why -OH group in phenol is ortho and para directing in nature ?
Text Solution
|
- Write the equation of the reaction which takes place when l-propanol i...
Text Solution
|
- Write the following reactions : Phenol with zinc dust.
Text Solution
|
- How Phenol is converted to Benzene ?
Text Solution
|
- How does phenol react with: Sodium
Text Solution
|
- Write the structure of Benzene diazonium chloride
Text Solution
|
- HNO(3)
Text Solution
|
- How does phenol react with: Chloroform
Text Solution
|
- Acid anhydride on reaction with 1^@ amine gives
Text Solution
|
- How does phenol react with: Ammonia ?
Text Solution
|
- Write a test to distinguish alcohols from phenols.
Text Solution
|
- Complete the following :
Text Solution
|
- Write the IUAC name of the compound HCOOH
Text Solution
|
- What is Coupling reaction ?
Text Solution
|
- Explain SchottenBaumann reaction.
Text Solution
|
- Write esterification reaction.
Text Solution
|
- How alcohols react with (i) Carboxylic acids (ii) Acid anh...
Text Solution
|