Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS , PHENOLS AND ETHERS
BETTER CHOICE PUBLICATION|Exercise 11. 5 SOME COMMERCIALLY IMPORTANT ALCOHOLS |9 VideosALCOHOLS , PHENOLS AND ETHERS
BETTER CHOICE PUBLICATION|Exercise 11.6 . ETHERS |8 VideosALCOHOLS , PHENOLS AND ETHERS
BETTER CHOICE PUBLICATION|Exercise 11 . 4. 2 PHYSICAL PROPERTIES |10 VideosALTERNATING CURRENTS
BETTER CHOICE PUBLICATION|Exercise MOST EXPECTED QUESTIONS|11 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ALCOHOLS , PHENOLS AND ETHERS -11. 4 . 3 . CHEMICAL PROPERTIES
- How alcohols react with (i) Carboxylic acids (ii) Acid anh...
Text Solution
|
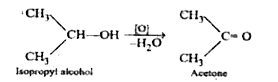
- Discuss oxidation of primary,secondary and tertiary alcohols.
Text Solution
|
- Discuss oxidation of primary,secondary and tertiary alcohols.
Text Solution
|
- How will you convert ethanol to ethanoic acid ?
Text Solution
|
- What happens when phenol is treated with conc . HNO(3) ?
Text Solution
|
- How is phenol converted into picric acid ?
Text Solution
|
- What is Fries Rearrangement .
Text Solution
|
- Write the following reactions : Phenol with phthallic anhydride.
Text Solution
|
- How alcohols react with SOCl(2)
Text Solution
|
- How alcohols react with PCl(5)
Text Solution
|
- Write the structure of penta-1,3-diene
Text Solution
|
- Explain Lucas test to distinguish between 1^@, 2^@ and 3^@ alcohols.
Text Solution
|
- How will you distinguish primary, secondary and tertiary alcohols by d...
Text Solution
|
- Explain Victor Meyer’s test for secondary (2^@) alcohol.
Text Solution
|
- How will you distinguish secondary and tertiary alcohols are pas...
Text Solution
|
- How will you distinguish secondary and tertiary alcohols are pas...
Text Solution
|
- How will you distinguish primary, secondary and tertiary alcohols by d...
Text Solution
|
- Write the reaction of primary , secondary and tertiary alcohols w...
Text Solution
|
- Writing chemical equations What happen when phenol is treated with...
Text Solution
|
- How will you convert ethanol to ethane ?
Text Solution
|