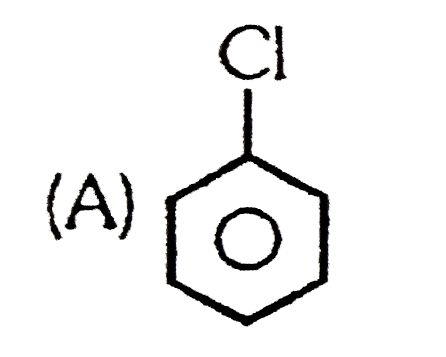
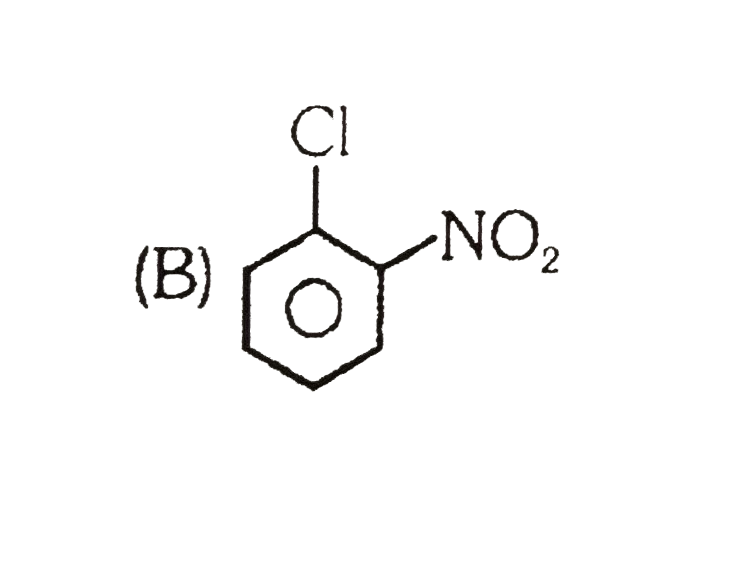
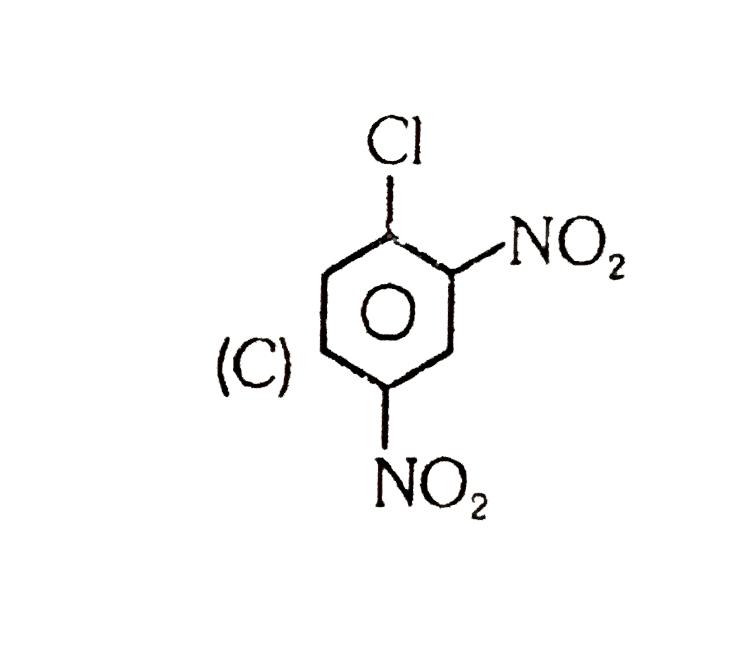
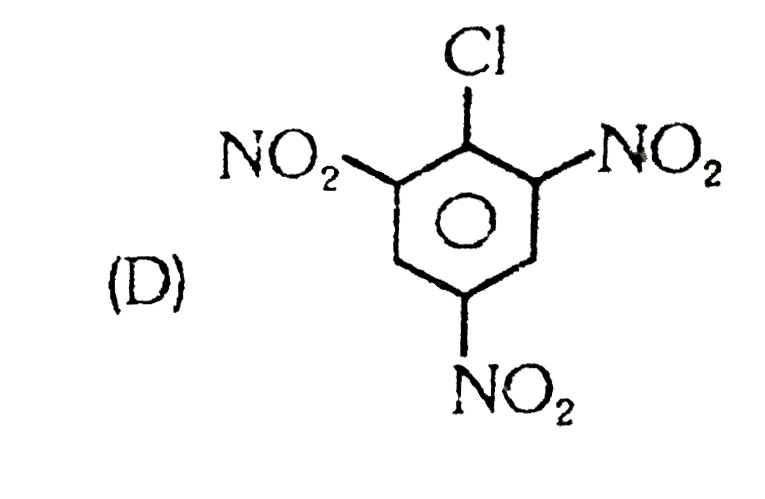
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ELECTROCHEMISTRY-EXERCISE -05 [B]
- Which of the following undergoes Hydrolysis most easily:
Text Solution
|
- Find the solubility product of a saturated solution of Ag(2)CrO(4) in ...
Text Solution
|
- Calculate the equilibrium constant for the reaction, 2Fe^(3+) + 3I^(-)...
Text Solution
|
- The gasX at 1 atm is bubbled through a solution containing a mixture o...
Text Solution
|
- The following electrochemical cell has been set up Pt(1)|Fe^(3+)|Fe^...
Text Solution
|
- Copper sulphate solution (250 ML) was electrolyzed using a platinum an...
Text Solution
|
- For the electrochemical cell, (M|M^(o+))||(X^(c-)|X),E^(c-).((M^(o+)|M...
Text Solution
|
- The standard potential of the following cell is 0.23V at 15^(@)C and 0...
Text Solution
|
- A standard solution of KNO(3) is used to make salt bridge, because
Text Solution
|
- The correct order of equivalent conductance at infinite dilution of ...
Text Solution
|
- The reaction 3ClO^(ө)(aq)rarrClO(3)^(-)(aq)+2Cl^(ө)(aq) is an exam...
Text Solution
|
- Complete the following chemical reaction equations : (i)MnO(4)^(...
Text Solution
|
- Two students use same stock solution of ZnSO(4) and a solution of CuSO...
Text Solution
|
- In an electrolytic cell, the flow of electrons is form
Text Solution
|
- Find the equilibrium constant of the following reaction Cu^(2+) (a...
Text Solution
|
- Zn|Zn^(2+)(a=0.1M)||Fe^(2+)(a=0.01M)Fe. The EMF of the above cell i...
Text Solution
|
- Calculate DeltaG(r)^(@) of the following reaction Ag^(+)(aq)+cI^(-)(...
Text Solution
|
- The rusting of iron takes place as follows : 2H^(o+)+2e^(-) +(1)/(2...
Text Solution
|
- We have taken a saturated solultion of AgBr, K(SP) is 12 xx 10^(-4). I...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|