A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ALKYL AND ARYL HALIDE-EXERCISE
- The basicity of RO^(-),HO^(-),RCOO^(-),ROH and H(2)O are of the order-
Text Solution
|
- Which of the following are aprotic solvents:
Text Solution
|
- Which is/are true statements (s):
Text Solution
|
- Ph-overset(Me)overset(|)underset(H)underset(|)(C)-OH overset(SOCl(2))u...
Text Solution
|
- Which of the following undergoes Hydrolysis most easily:
Text Solution
|
- A compound 'A' formula of C(3)H(6)Cl(2) on reaction with alkali can gi...
Text Solution
|
- Isobutyl magneisum bromide with dry ether and absolute alcohol gives
Text Solution
|
- Following reaction is
Text Solution
|
- On treatment with chlorine in presence of sunlight toluene gives the p...
Text Solution
|
- In S(N^(1)) reaction an optically active substrates mainly gives
Text Solution
|
- Alkyl iodide can be prepared by:-
Text Solution
|
- Which of the following reagents can be used to prepare and alkyl halid...
Text Solution
|
- Which of the following reactions depict the nucleophilic substitition ...
Text Solution
|
- For an S(N^(2)) reaction which of the following statements are true:-
Text Solution
|
- Which of the following is an S(N^(2)) reaction:-
Text Solution
|
- Match the following.
Text Solution
|
- Match the following.
Text Solution
|
- Math the following.
Text Solution
|
- Statement-I: Iodination of akanes is carried out by heat in presence o...
Text Solution
|
- Statement-I: Chloropropane has higher boiling point than chloroethane....
Text Solution
|
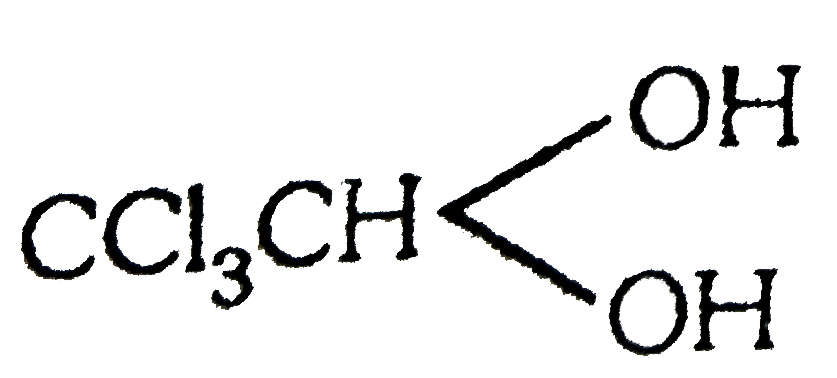 show H-Bonding
show H-Bonding