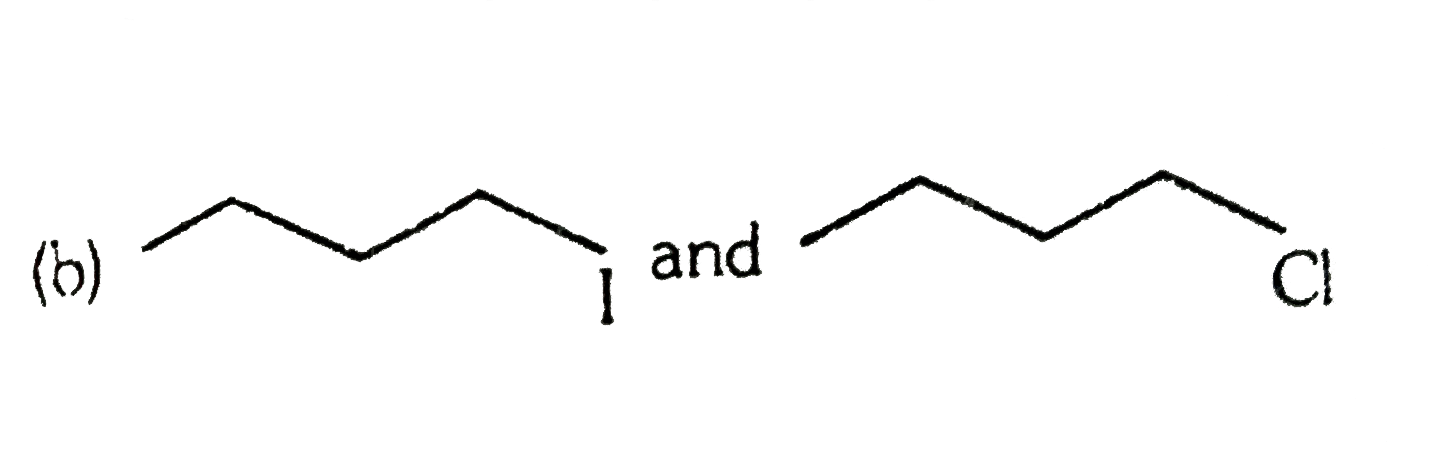
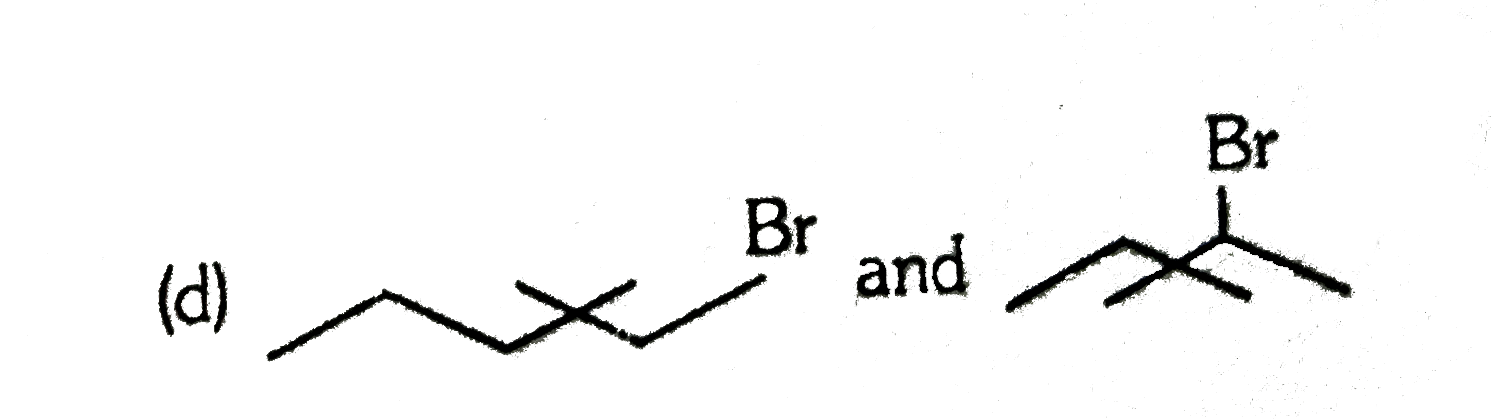
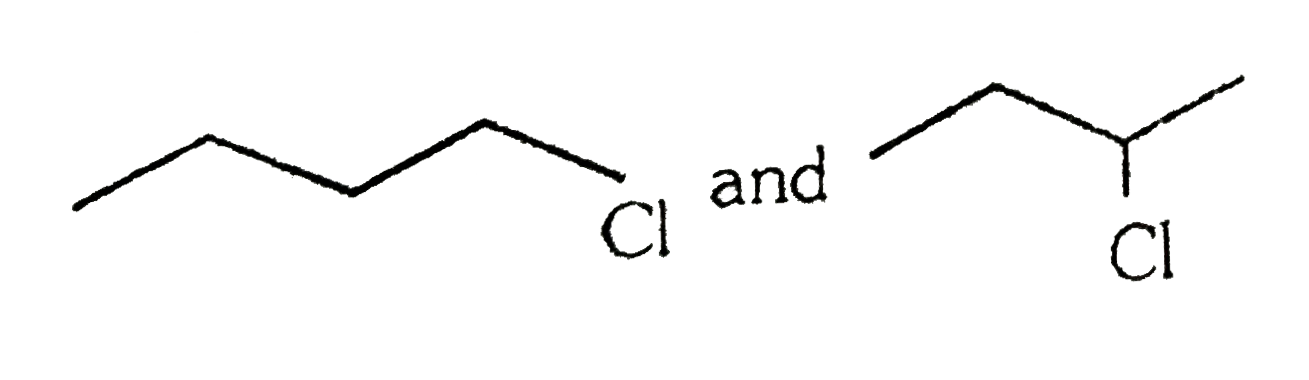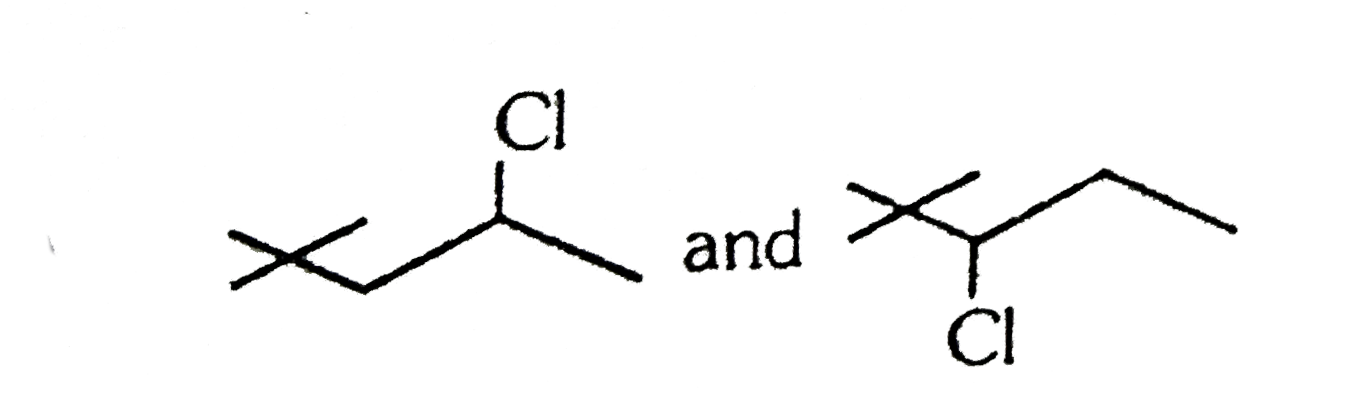A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ALKYL AND ARYL HALIDE-EXERCISE
- There is an overall 29-fold difference in reactivity of 1-chlorohexane...
Text Solution
|
- Identify the product when A reacts with
Text Solution
|
- Which is faster in the following pairs of halogen compounds undergoing...
Text Solution
|
- R -Mg-Br(A) on reaction with H(2)O forms a gas (B), which occupied 1.4...
Text Solution
|
- Provide structure of major product in the following reaction indicatin...
Text Solution
|
- Propose mechanism of the following reactions:
Text Solution
|
- Which of the following alkyl halide could be successfully used to synt...
Text Solution
|
- An alkyl bromide A has molecular formula C(8)H(17)Br and four differen...
Text Solution
|
- Identify A to G in the following. (a) overset(Br(2), C Cl(4))rarr A...
Text Solution
|
- Identify B to F
Text Solution
|
- Vinyl chloride does not give S(N) reaction but allyl chloride gives. E...
Text Solution
|
- Arrange the following in the increasing order of their ability as a le...
Text Solution
|
- RBr when treated with AgCN in a highly polar solvent gives RNC whereas...
Text Solution
|
- SN^(1) reaction is feasable in-
Text Solution
|
- Bottles containing C6H5I and C6H5CH2I lost their original labels. They...
Text Solution
|
- The reaction of chloroform with alcoholic KOH and p-toluidine form-
Text Solution
|
- The compound formed on heating chlorobenzene with chloral in the pres...
Text Solution
|
- Tertiary alkyl halide are practically inert to substitution by SN^(2)...
Text Solution
|
- Among the following the one that gives positive iodoform test upon rea...
Text Solution
|
- The structure of the major product formed in the following reaction is...
Text Solution
|