Explain the following observations:
(a) Azide ion `(N_(3))` react with `2-` bromopentane thousand times faster than with neopentyl bromide in a `S_(N)2` reaction through former is a secondary halide while latter is primary.
(b) What will happen to the stereochemistry of product of the following reaction:
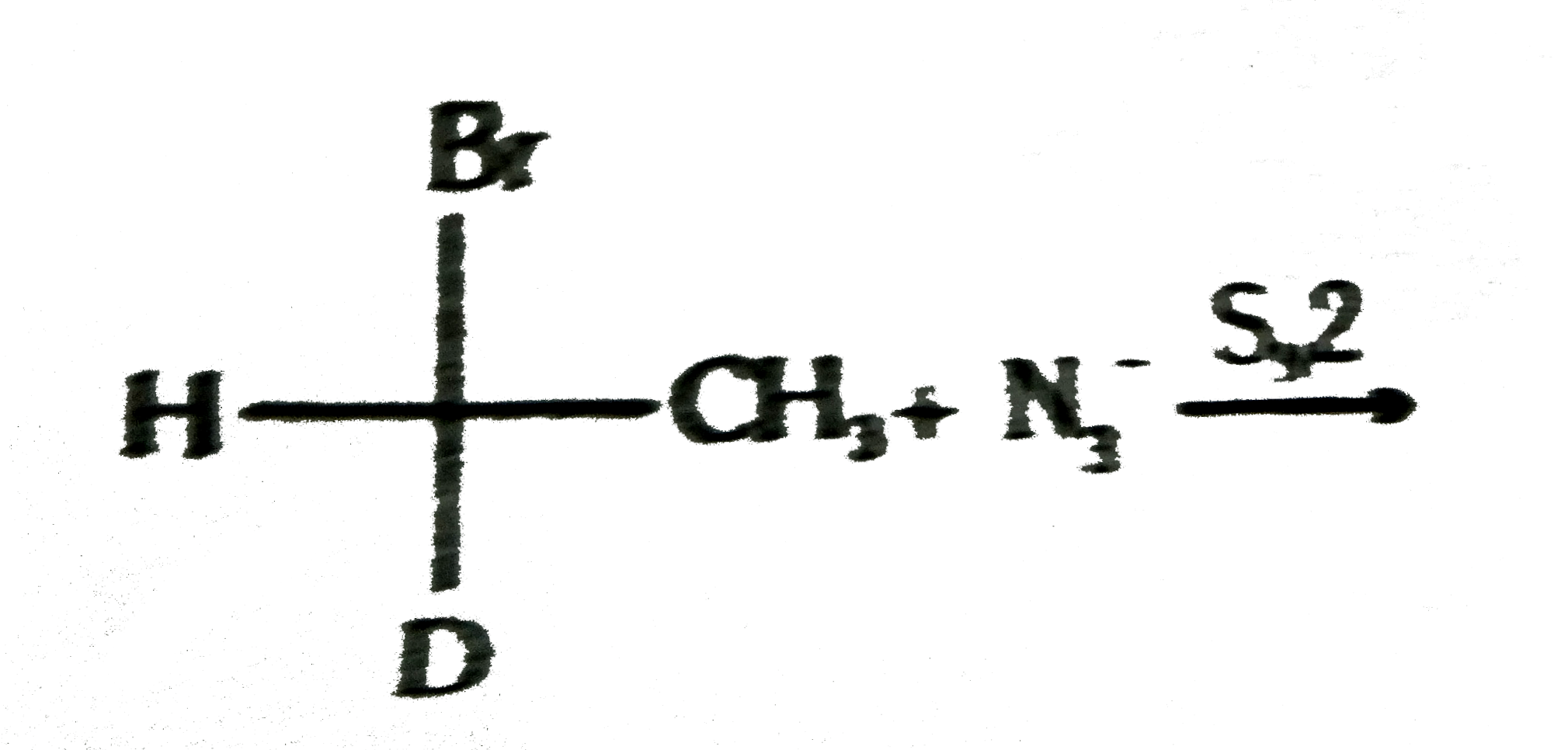
(c) What will happens to the rate if the concentration of alkyl bromide in (b) is doubled?
(d) Wha will happen to the rate if the concentration of azide ion in (b) is doubled?
(e) How the sign of optical rotation of reactant and product are related in (b)
(f) When allowed to stand in dilute `H_(2)SO_(4)` laevo-rotatory 2-butanol slowly loss optical activity.