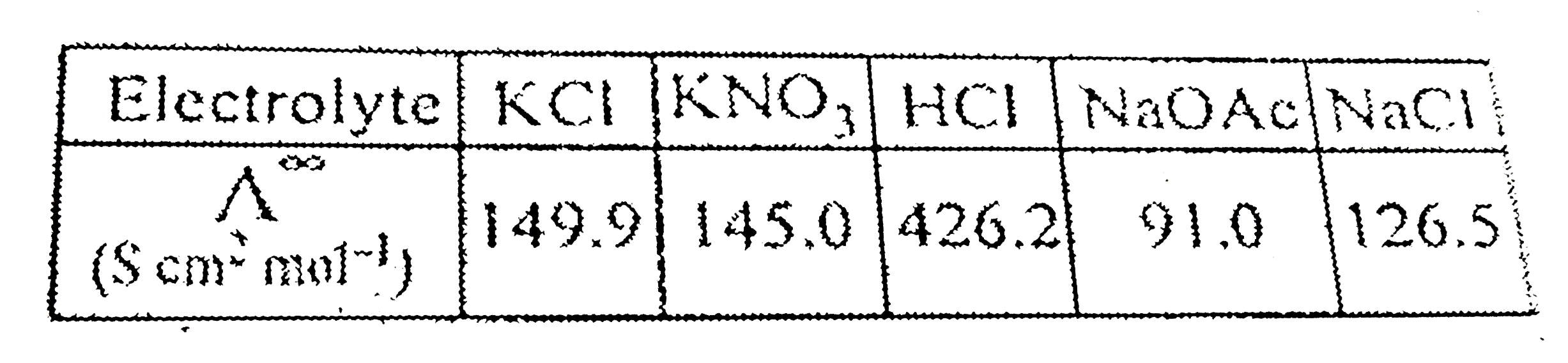A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ELECTROCHEMISTRY-EXERCISE-05 [A]
- The standard e.m.f of a cell, involving one electron change is found t...
Text Solution
|
- Consider the following E^(@) values E^(@) values E(Fe^(3+)//Fe^(2+))^...
Text Solution
|
- Calculate A(HOAC)^(oo) using appropriate molar conductances of the éle...
Text Solution
|
- The highest electrical conductivity of the following aqueous solutions...
Text Solution
|
- Aluminium oxide may be electrolysed at 1000^(@)C to furnish aluminium ...
Text Solution
|
- For a spontaneous reaction, DeltaG, equilibrium constant K and E("cell...
Text Solution
|
- The molar conductivities Lambda(NaOAc)^@ and Lambda(HCI)^@ at infinite...
Text Solution
|
- Resistance of a conductivity cell filled with 0.1 mol L^(-1) KCl solut...
Text Solution
|
- At 25^(@)C, {:(Ag+I^(-)rarr,AgI+e,,E^(@) =0.152V),(Agrarr,Ag.^(+)+e,,E...
Text Solution
|
- The cell, Zn | Zn^(2+) (1M) || (1M) | Cu (E(cell)^(@) = 1.10V), was wa...
Text Solution
|
- Given E(Cr^(3+)//cr)^@ =- 0.72 V, E(Fe^(2+)//Fe)^@ =- 0.42 V. The pote...
Text Solution
|
- Given : E(Fe^(3+)//Fe)^(@) = -0.036V, E(FE^(2+)//Fe)^(@)= -0.439V. The...
Text Solution
|
- The Gibbs energy for the decomposition of Al2O3 at 500^@C is as follow...
Text Solution
|
- Resistance of 0.2 M solution of an electrolyte is 50Omega. The specifi...
Text Solution
|
- The reduction potential of hydrogen half cell will be negative if :
Text Solution
|
- The standard reduction potentials for Zn^(2+)//Zn, Ni^(2+)//Ni, and Fe...
Text Solution
|
- Use the data given in Q.8 and find out which of the following is the ...
Text Solution
|
- The equivalent conductance of NaCl at concentration C and at infinite...
Text Solution
|
- In which of the following reactions H(2)O(2) acts as a reducing agent?...
Text Solution
|
- Resistance of 0.2 M of solution of an electrolysis is 50 Omega. The sp...
Text Solution
|