Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALLEN-SOLUTIONS-EXERCISE-04 [A]
- At vapour pressures of ethanol and metanol are 44.5 mm Hg and 88.7 mm ...
Text Solution
|
- The vapour pressure of pure benzene at a certanin temperature is 640 m...
Text Solution
|
- The addition of 0.643 g of a compound to 50 mL of benzene (density 0.8...
Text Solution
|
- A solution containing 30 g of a non-volatile solute in exactly 90 g of...
Text Solution
|
- The freezing point of ether was lowered by 0.60^(@)Con dissolving 2.0g...
Text Solution
|
- The addition of 3g of a substance to 100g C CI(4)(M = 154 g mol^(-1)) ...
Text Solution
|
- To 500 cm^(3) of water, 3.0xx10^(-3) kg of acetic acid is added. If 23...
Text Solution
|
- 0.01 m aqueous solution of K(3)[Fe(CN)(6)] freezes at -0.062^(@)C. Wha...
Text Solution
|
- The degree of dissociation of Ca(NO(3))(2) in a dilute aqueous solutio...
Text Solution
|
- A solution containing 0.11kg of barium nitrate in 0.1kg of water boils...
Text Solution
|
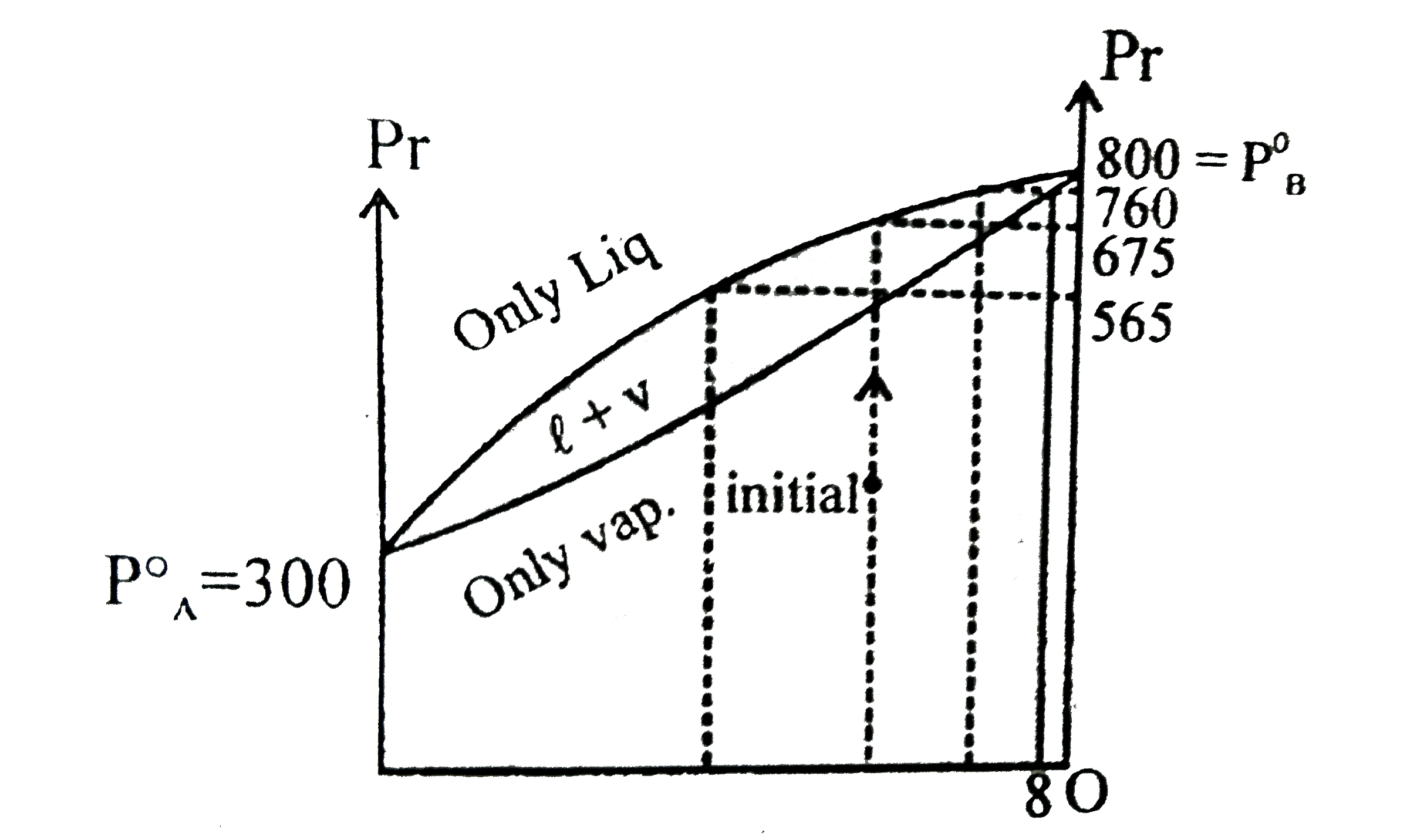
- The vapour pressure of two pure liquids A and B that forms an ideal so...
Text Solution
|
- Sea water is found to contain 5.85% NaCl and 9.50% MgCl(2) by weight o...
Text Solution
|
- Find the freezing point of a glucose solution whose osmotic pressure a...
Text Solution
|
- The latent heat of fusion of ice is 80 calories per gram at 0^(@)C. Wh...
Text Solution
|
- At 300K, two solutions of glucose in water of concentration 0.01M and ...
Text Solution
|
- At 10^(@)C, the osmotic pressure of urea solution is 500 mm.The soluti...
Text Solution
|
- An M/10 solution of potassium ferrocyanide is 46% dissociated at 300 K...
Text Solution
|