A
B
C
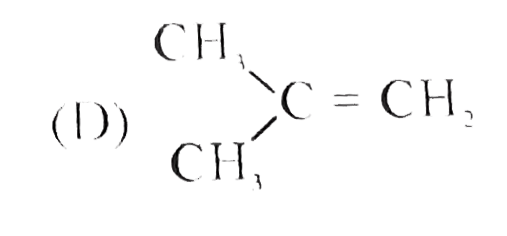
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Alkenes undergo electrophilic addition reactions. Electrophilic additi...
Text Solution
|
- Alkenes undergo electrophilic addition reactions. Electrophilic additi...
Text Solution
|
- Alkenes undergo electrophilic addition reactions. Electrophilic additi...
Text Solution
|
- Assertion (A): Electrophilic addition addition of Br(2) to alkyne proc...
Text Solution
|
- Statement-I : Alkyness are more reactive than alkenes towards electrop...
Text Solution
|
- Which of the following alkenes is expected to be more reactive towards...
Text Solution
|
- Why do alkenes undergo electrophilic addition reactions ?
Text Solution
|
- Why do alkenes undergo electrophilic addition reactions ?
Text Solution
|
- A : Alkynes is more reactive than alkene towards electrophilic additio...
Text Solution
|