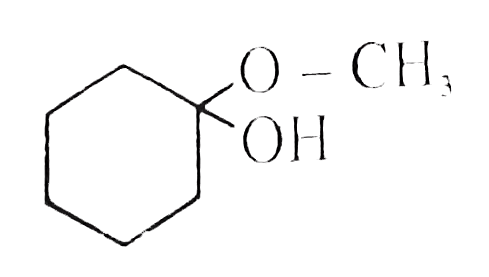
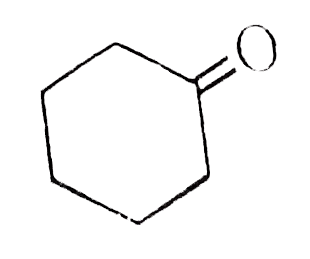
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Alkenes undergo electrophilic addition reactions. Electrophilic additi...
Text Solution
|
- Alkenes undergo electrophilic addition reactions. Electrophilic additi...
Text Solution
|
- Alkenes undergo electrophilic addition reactions. Electrophilic additi...
Text Solution
|
- Electrophillic addition reaction proceed in two steps. The first step ...
Text Solution
|
- Electrophilic additions reactions proceed in two steps. The first step...
Text Solution
|
- Electrophilic additions reactions proceed in two steps. The first step...
Text Solution
|
- Electrophilic addition reactions proceed in two steps. The first step ...
Text Solution
|
- Mechanism of acid catalysed hydration reaction involves (i) Protonat...
Text Solution
|
- Assertion (A): addition of HCN in alkene is a type of electrophilic su...
Text Solution
|
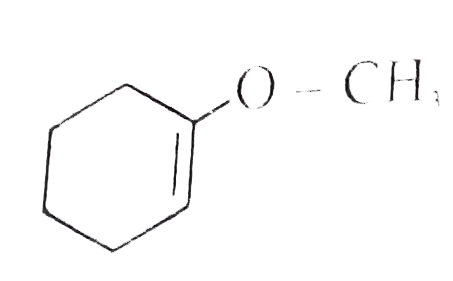 `+H_(2)O overset(H_(2)SO_(4))rarr` Product
`+H_(2)O overset(H_(2)SO_(4))rarr` Product