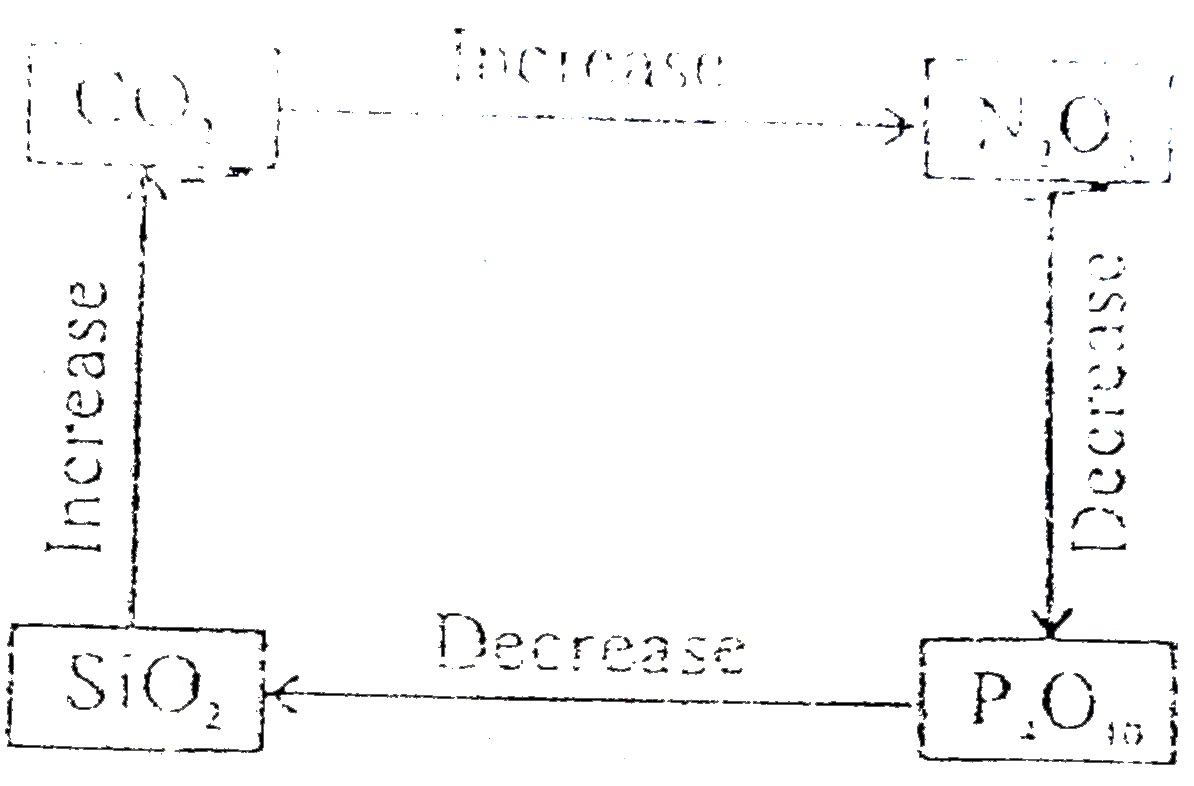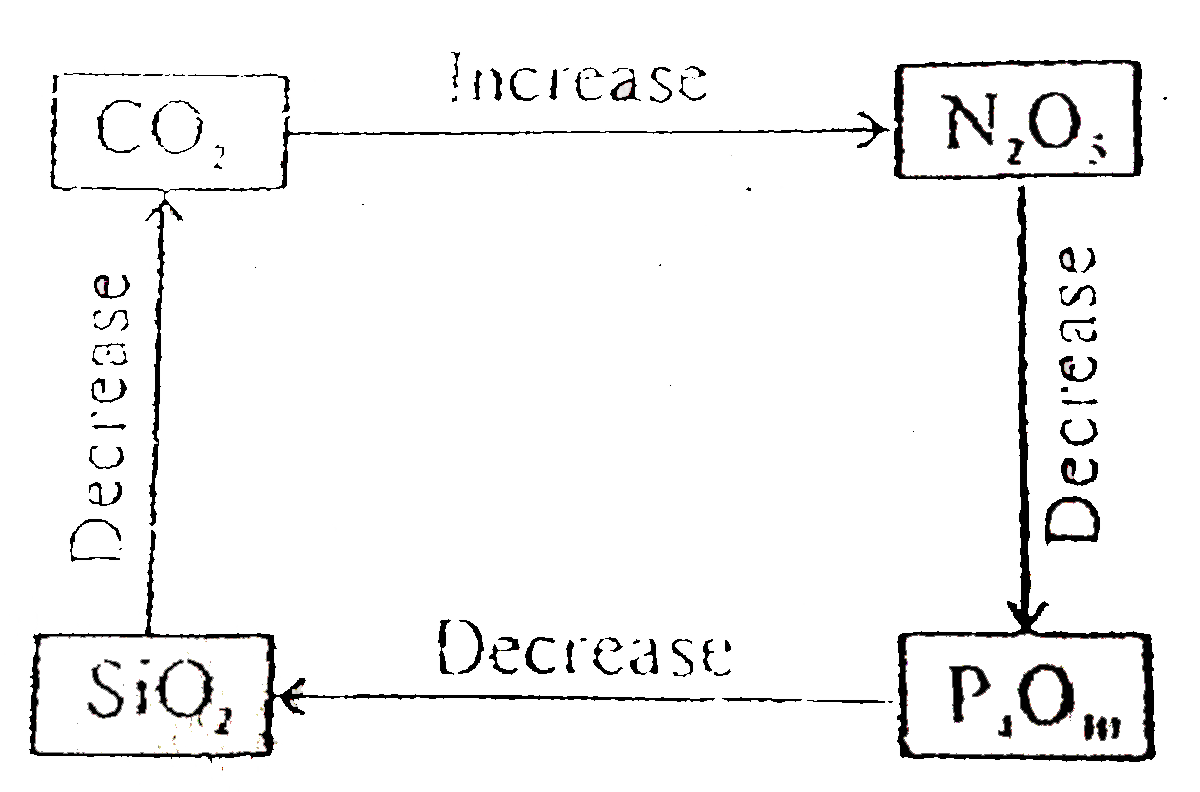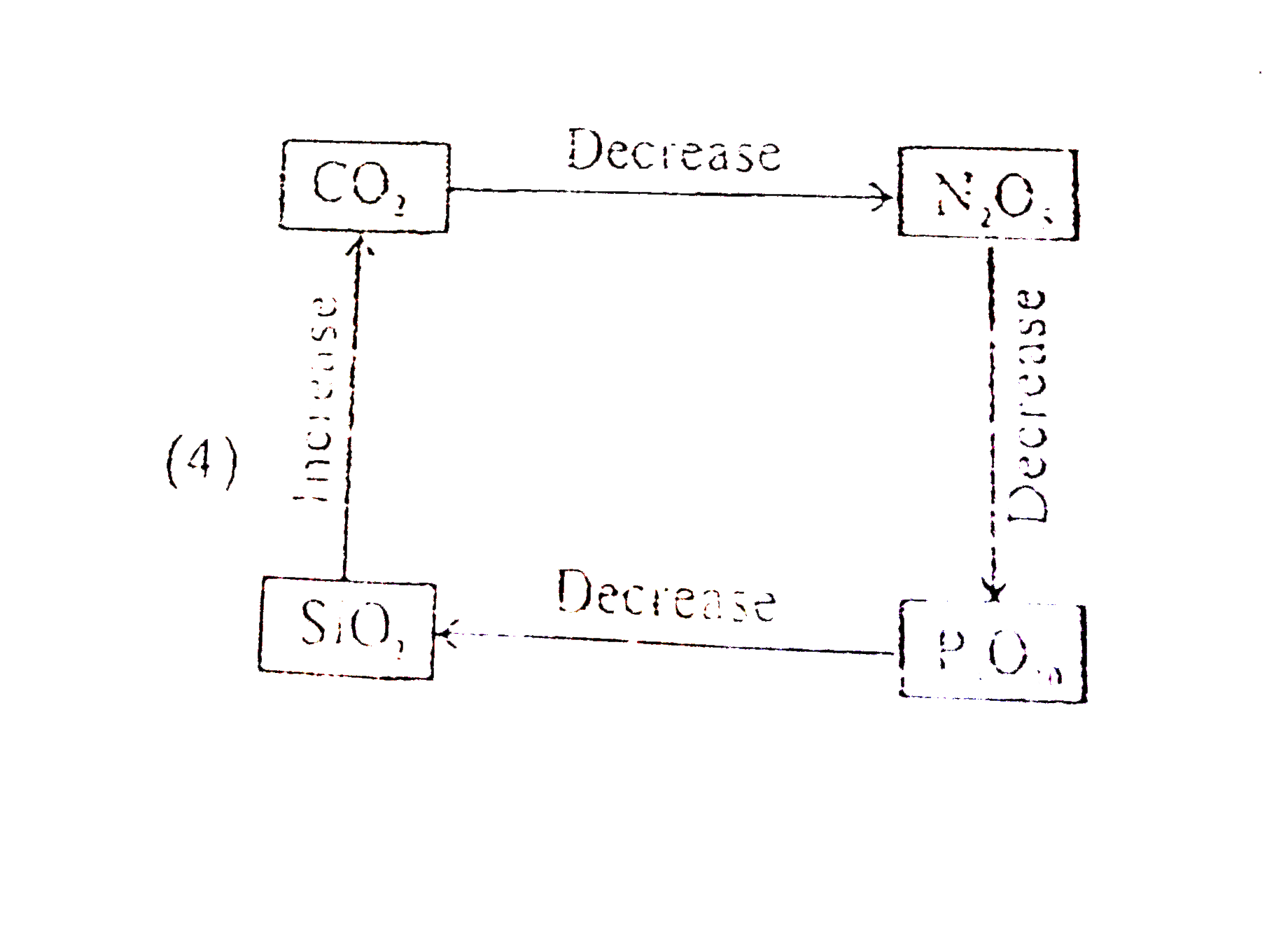A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Select correct diagram about the acidic strength of oxides :-
Text Solution
|
- Select correct order about bond strength
Text Solution
|
- The correct of increasing acidic strength is .
Text Solution
|
- The correct decreasing order for the acidic strength of oxides of nitr...
Text Solution
|
- Ellingham diagram is given below for the formation of some oxides. The...
Text Solution
|
- Ellingham diagram is given below for the formation of some oxides. The...
Text Solution
|
- Correct order of acidic strength :
Text Solution
|
- ऑक्सीकारक की प्रबलता का सही क्रम है :
Text Solution
|
- हैलोजन की ऑक्सीकरण संख्या में ……….. के साथ हैलोजन के विभिन्न ऑक्सोअम्ल...
Text Solution
|