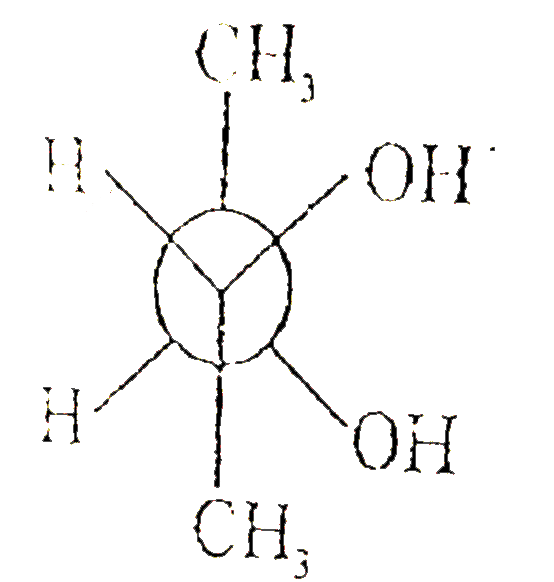
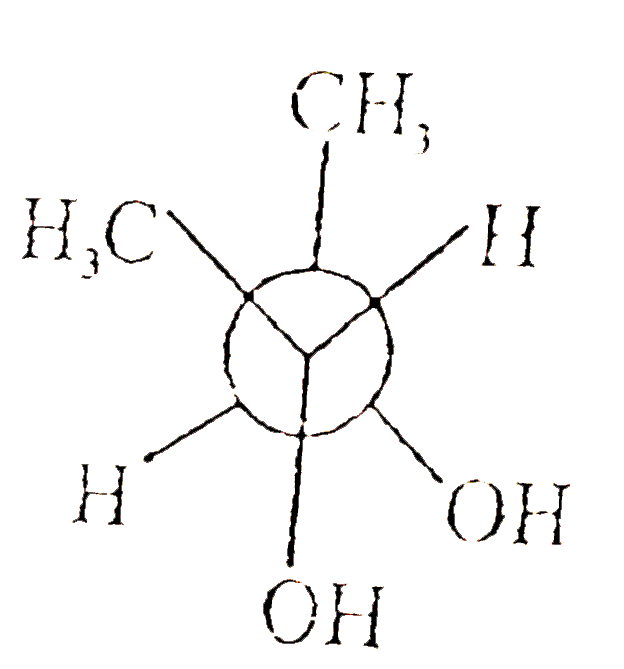
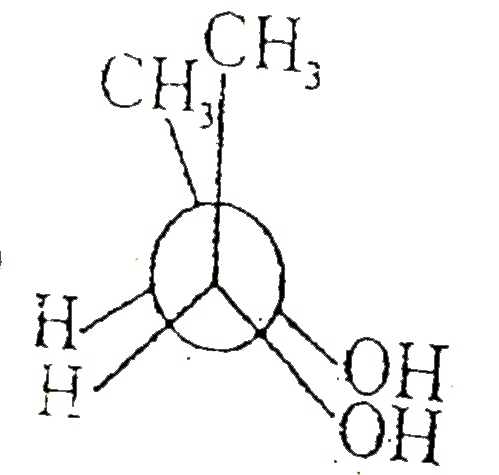
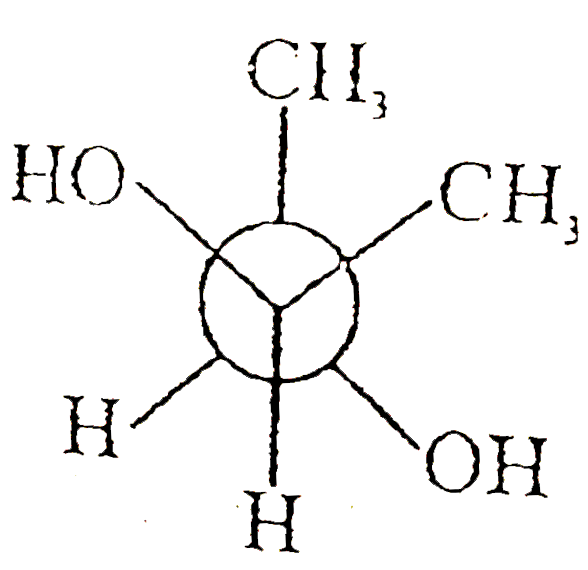
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-TEST PAPER-Exercise (Chemistry)
- The IUPAC name of compound is :-
Text Solution
|
- Arrange the following alkenes in order of their stability
Text Solution
|
- are related to each other form?
Text Solution
|
- Which of the following expected to have 100% enolic form?
Text Solution
|
- Which of the following is most stable confirmational isomer :-
Text Solution
|
- Which of the following is not a valid resonance structure of the other...
Text Solution
|
- The order of acidic strength for the following compounds is :-
Text Solution
|
- Arrange the following in decreasing order of basic strenth. underset...
Text Solution
|
- Match the following given columns:-
Text Solution
|
- The maximum number of identical bond angle in CH(2)Cl(2) are :-
Text Solution
|
- Configration of x,y and z are given - X=[Ne]3s^(2)3p^(5), Y=[Ne]3s^...
Text Solution
|
- In allene (C(3)H(4)), the type(s) of hybridization of the carbon atoms...
Text Solution
|
- Correct order of strength of a pi-bond(s) are :- (i) BO(3)^(3-)gtCO(...
Text Solution
|
- Solubility and thermal stability both increase drown the group for (A....
Text Solution
|
- Match the columns :- Correct option is :-
Text Solution
|
- Total planes containing maximum atoms in IF(7) will be :-
Text Solution
|
- Which of the following orders is correct? (1) SbH(3)gt NH(3) gt AsH(...
Text Solution
|
- The incorrect order of solubility in water is:
Text Solution
|
- The incorrect statement regarding 15 th group hydrides (EH(3))[E=N,P,A...
Text Solution
|
- Which of the following order of Bond strength is/are correct :- (a) ...
Text Solution
|