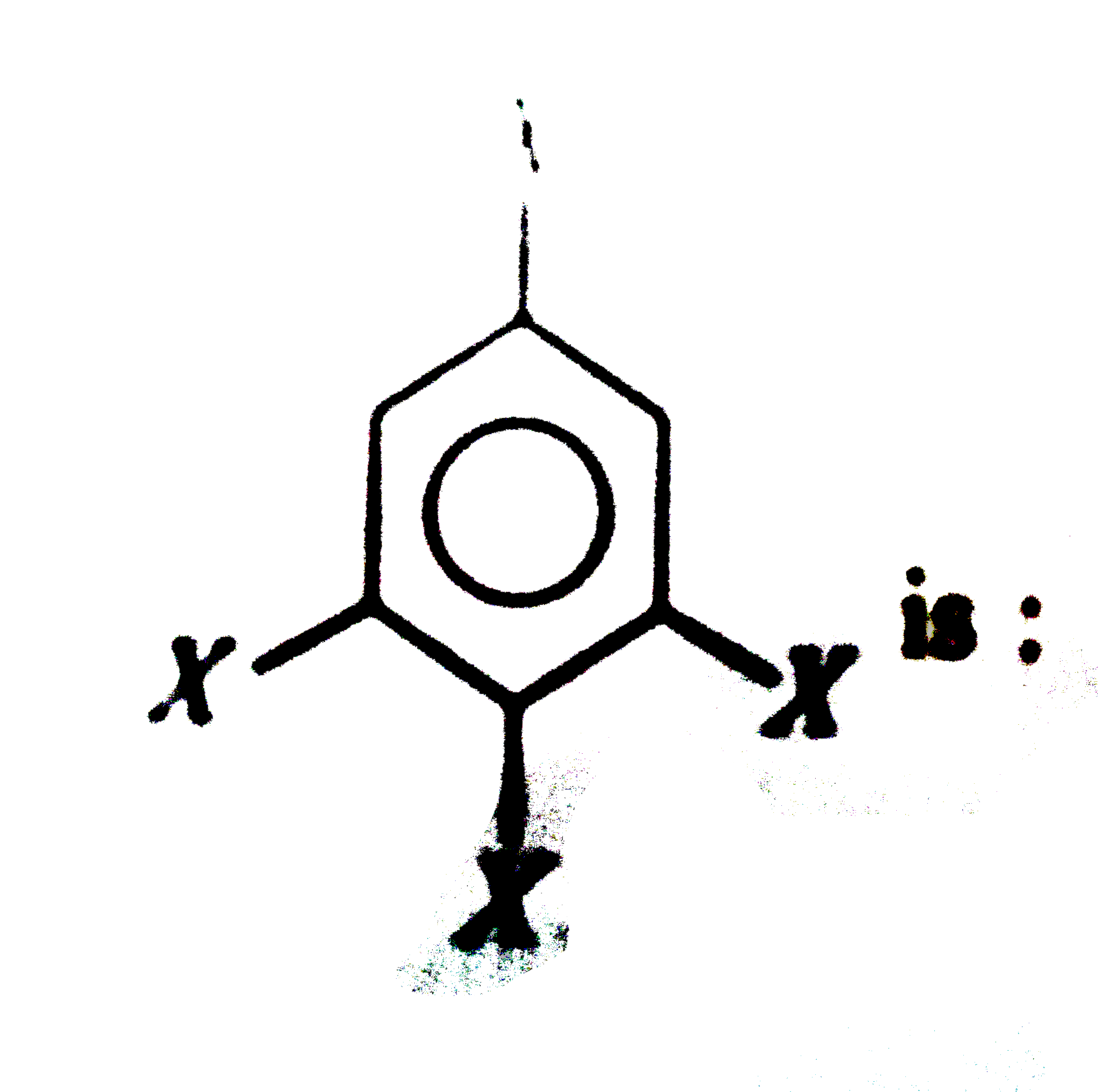A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-INORGANIC CHEMISTRY
- Which of the following represent most effective pi-bond
Text Solution
|
- In which of the following reactions hybridisation of underlined specie...
Text Solution
|
- Dipole moment of is 1.5D. The dipole moment of
Text Solution
|
- Which of the following compound has non-zero dipole moment
Text Solution
|
- Which of the following molecule is planar due to back bonding
Text Solution
|
- Amongst the following , molecule having maximum bond angles of 90^(@) ...
Text Solution
|
- Which of the following statement is incorrect
Text Solution
|
- The coordinate bond is absent in
Text Solution
|
- Which of the following is least stable ?
Text Solution
|
- The true statement from the following are
Text Solution
|
- The bond order for Ne2 and C2, respectively are
Text Solution
|
- Back Bonding
Text Solution
|
- The bond angle in H(2)O molecule is .. .....
Text Solution
|
- What is the order of stability of N(2) and its ions ?
Text Solution
|
- Which of the following have identical bond order?
Text Solution
|
- In XeF(2), XeF(4) and XeF(6) the number of lone pairs on Xe is respect...
Text Solution
|
- Which one of the following compounds is the most soluble in water?
Text Solution
|
- The correct order of N-O bond lengths in NO, NO2^- , NO3^- and N2O4 is
Text Solution
|
- The correct order of A-O-A bond angle of (A=H,F or Cl)
Text Solution
|
- There are some species given below :- {:((a)O(2)^(+),,,,(b)CO),((c)B...
Text Solution
|
 is 1.5D. The dipole moment of
is 1.5D. The dipole moment of