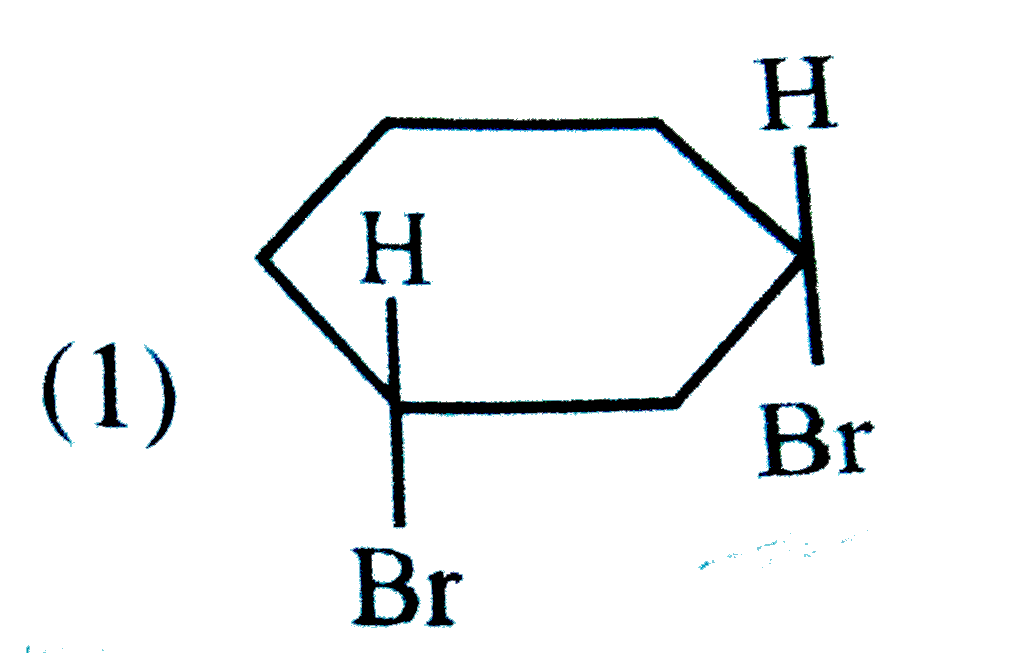
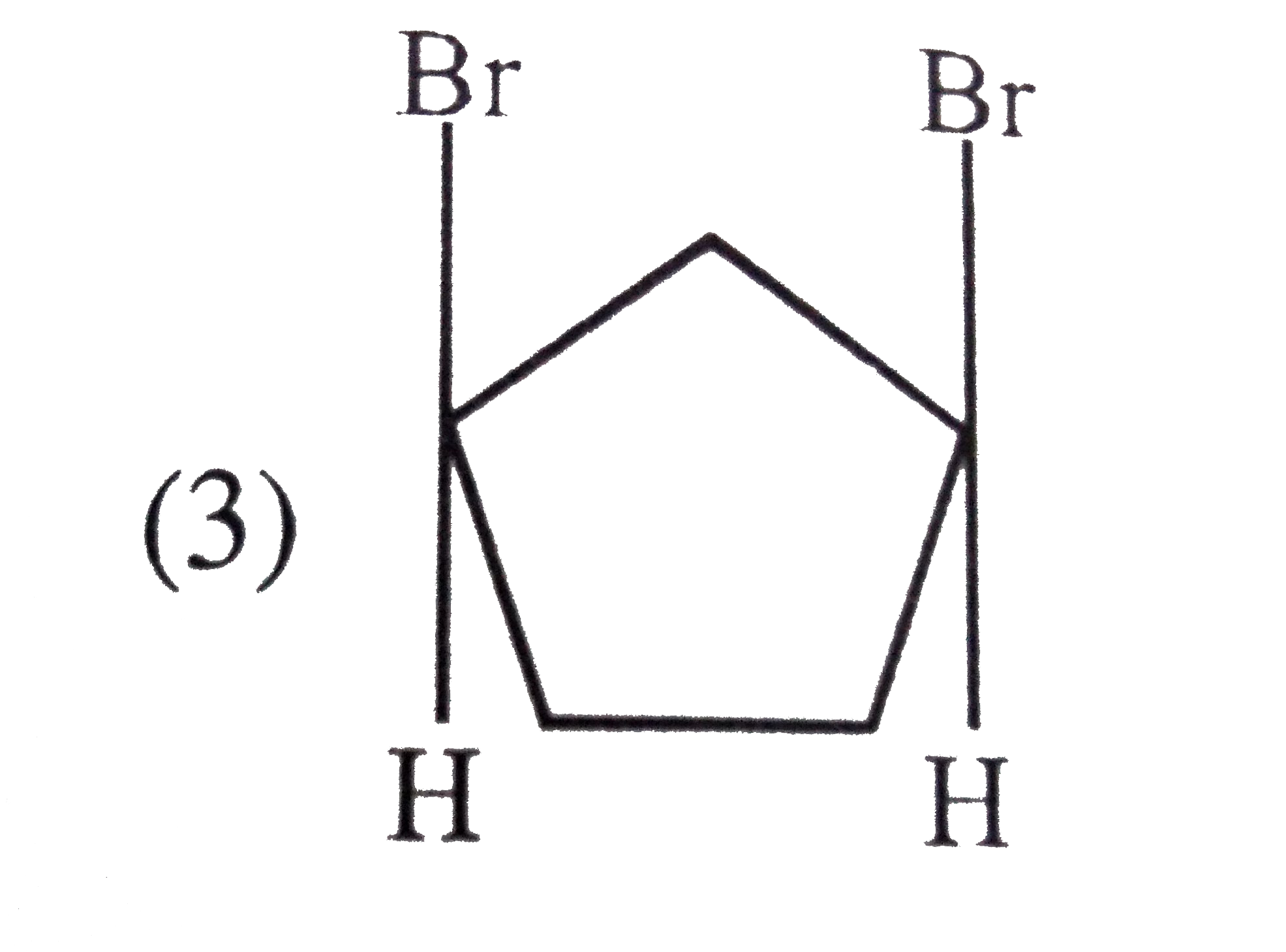
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- How many geometrical isomers of this compound are possible:
Text Solution
|
- Which of these molecule is optically active ?
Text Solution
|
- Which of the following compounds can non-superimposable mirror images?
Text Solution
|
- How many stereoisomers are possible in this compound ?
Text Solution
|
- Which of the following biphenyl is optical active:
Text Solution
|
- Which of the following is optically active
Text Solution
|
- Which of the following can be a meso compound
Text Solution
|
- The most stable conformation of Butane is
Text Solution
|
- Which of the following can show conformational isomerism:
Text Solution
|
- Number of stereoisomers of the given compound is CH(3)-CH=CH-underse...
Text Solution
|
- How many structures of aldehydes are possible for molecular formula ...
Text Solution
|
- Which of the following can show both tautomerism and optical isomerism
Text Solution
|
- Which of the following compound will show optical isomerism
Text Solution
|
- Which of the following molecule has non-superimposible mirror images ?
Text Solution
|
- What is relation between following compounds CH(3)-CH(2)-underset(CN...
Text Solution
|
- The E-isomer among the following is:
Text Solution
|
- One among the following will not show geometrical isomerism
Text Solution
|
- The optical inactivity of meso - tartaric acid is because of
Text Solution
|
- Which of the following will not be able to show optical isomerism (ena...
Text Solution
|
- The molecules represented by the following two structures are
Text Solution
|