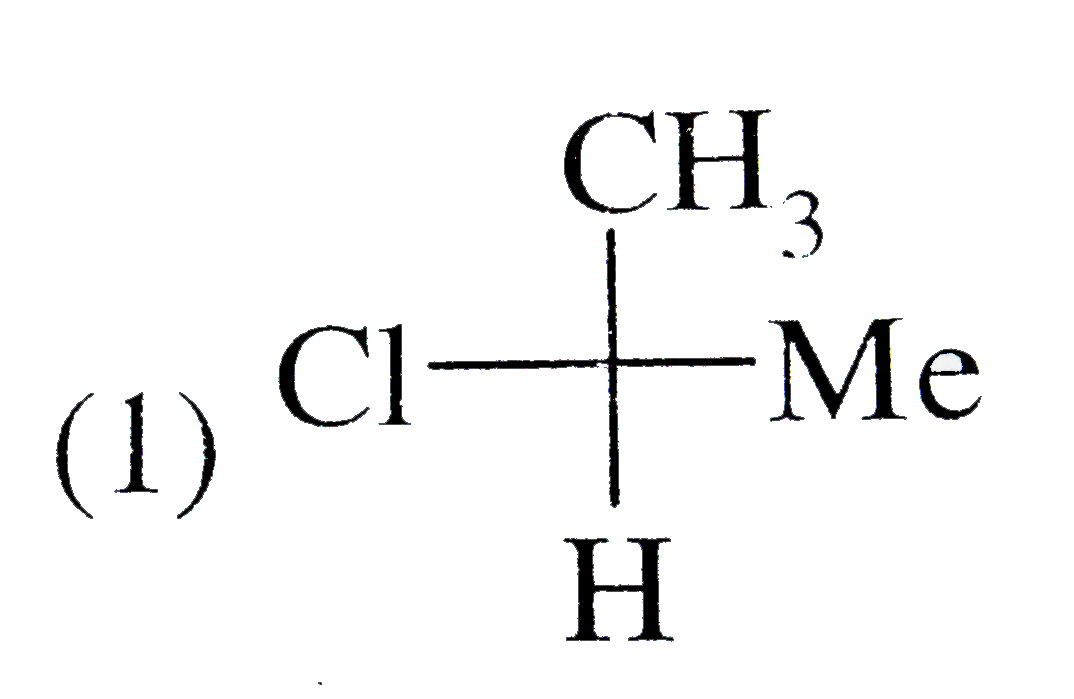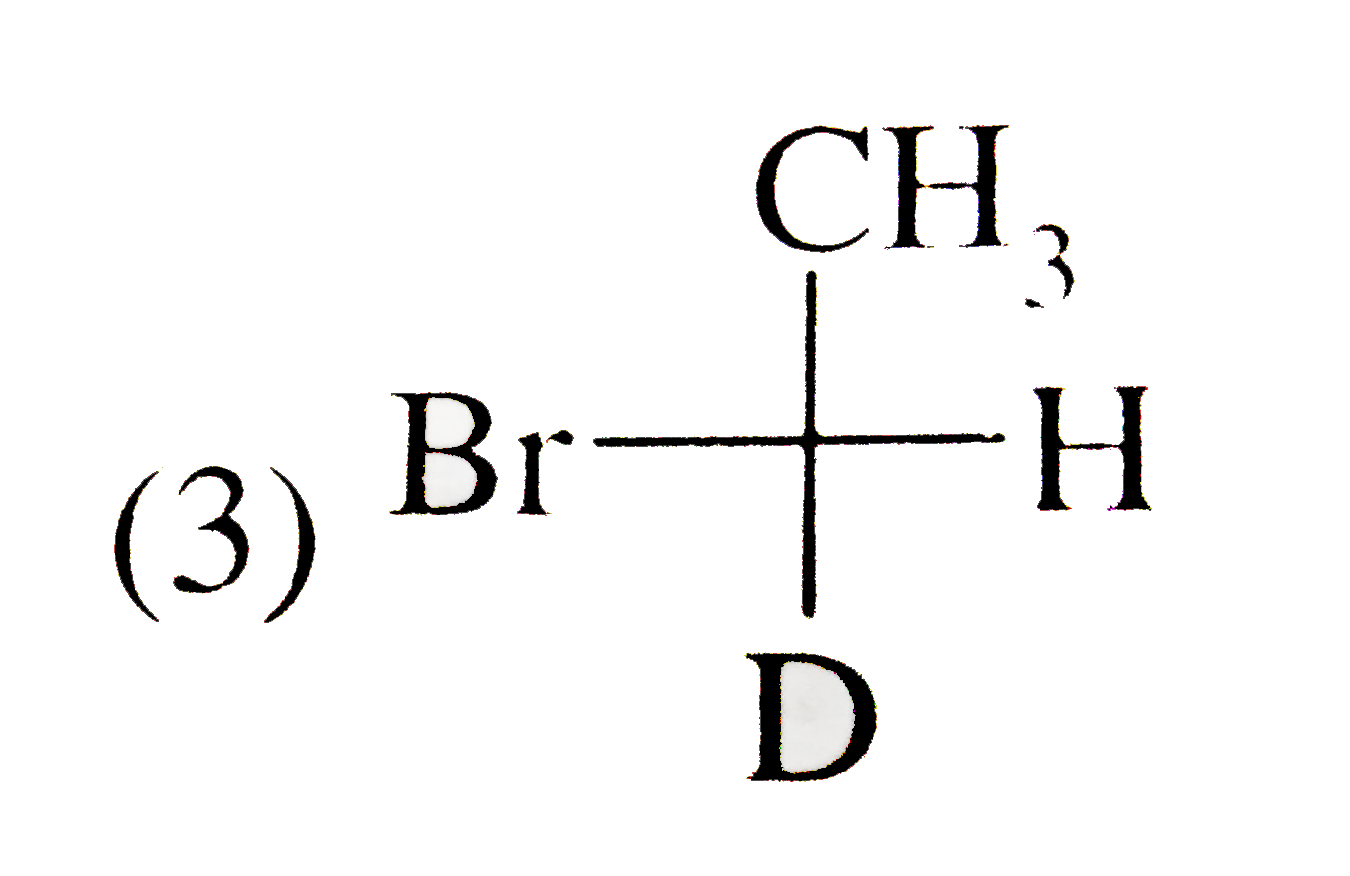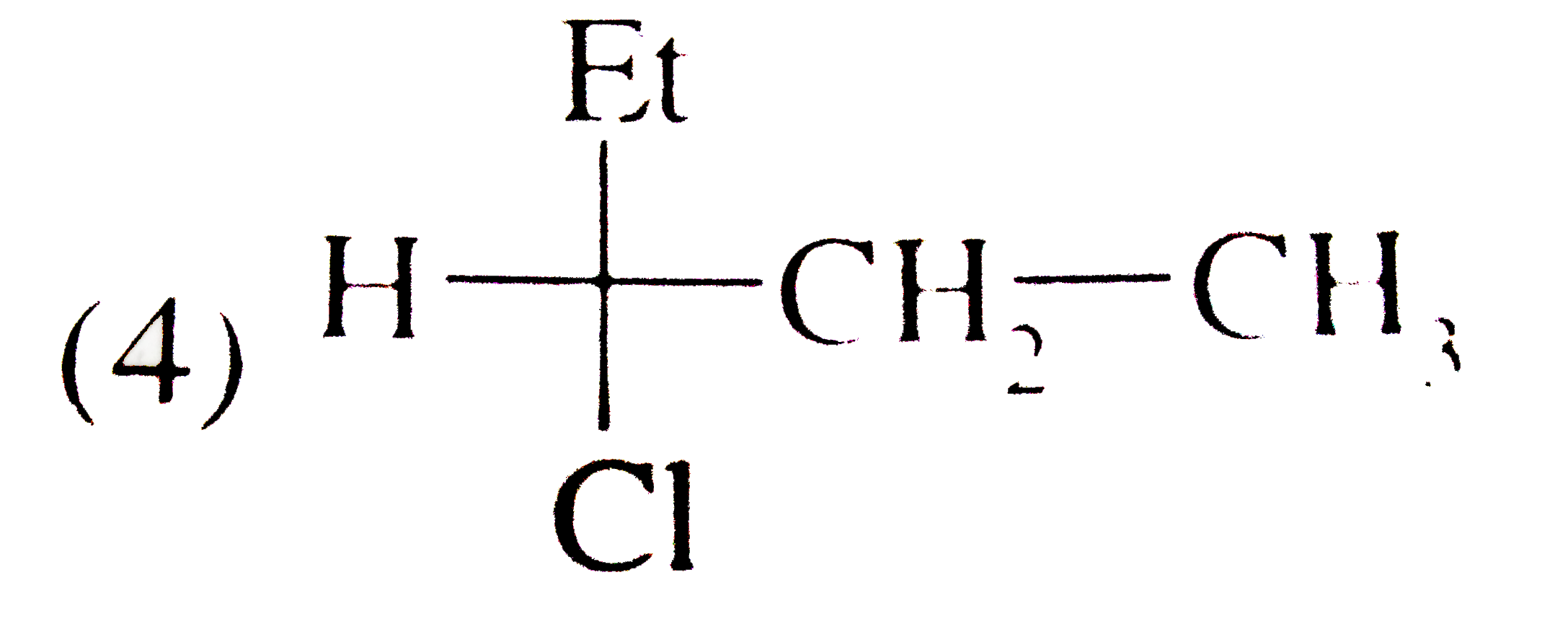A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- Which of the following can show both tautomerism and optical isomerism
Text Solution
|
- Which of the following compound will show optical isomerism
Text Solution
|
- Which of the following molecule has non-superimposible mirror images ?
Text Solution
|
- What is relation between following compounds CH(3)-CH(2)-underset(CN...
Text Solution
|
- The E-isomer among the following is:
Text Solution
|
- One among the following will not show geometrical isomerism
Text Solution
|
- The optical inactivity of meso - tartaric acid is because of
Text Solution
|
- Which of the following will not be able to show optical isomerism (ena...
Text Solution
|
- The molecules represented by the following two structures are
Text Solution
|
- Correct order of pKa is :
Text Solution
|
- Correct order of acidic strength:
Text Solution
|
- pK(a) increases in benzoic acid when substituent ''x'' is bonded at pa...
Text Solution
|
- Decreasing order of pKa is :
Text Solution
|
- Which of the following present the correct order of acidity in the giv...
Text Solution
|
- The correct order of K(a) is : (i) CH(3)-CH(2)-COOH (ii) OHC-CH(2)...
Text Solution
|
- Basic strength order will be :
Text Solution
|
- Decreasing order of stability of the following carbocation ? (A) m-(...
Text Solution
|
- Which of the following compound is Aromatic ?
Text Solution
|
- Correct order of pK(b) is (i) CH(3)-CH(2)-NH(2) (ii) Ph-CH(2)-NH(...
Text Solution
|
- Correct decreasing order of pK(b) is
Text Solution
|