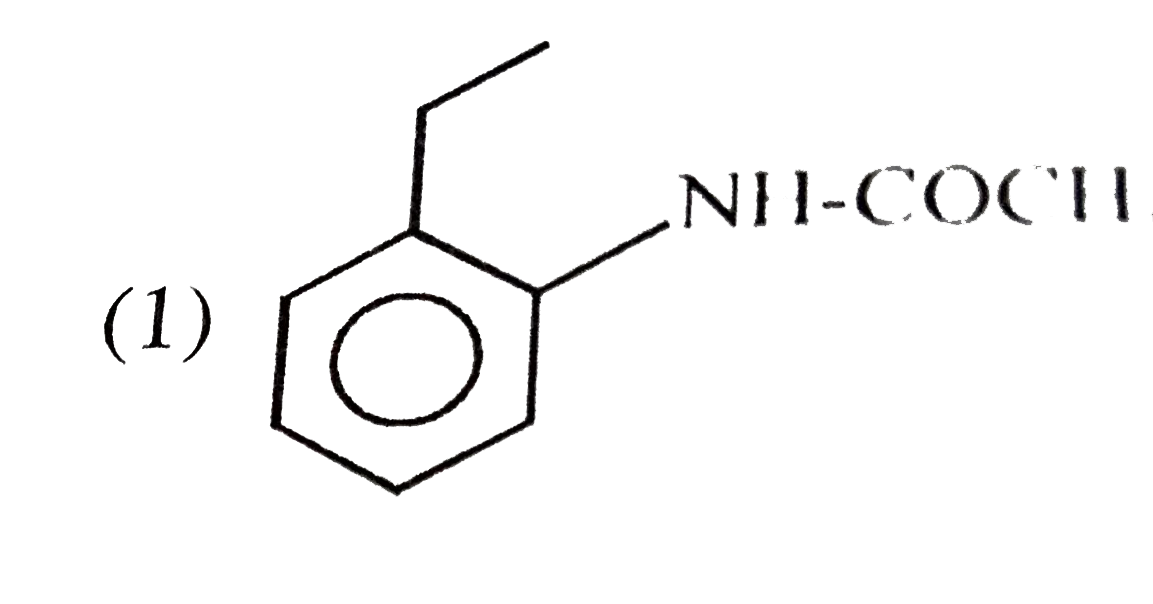
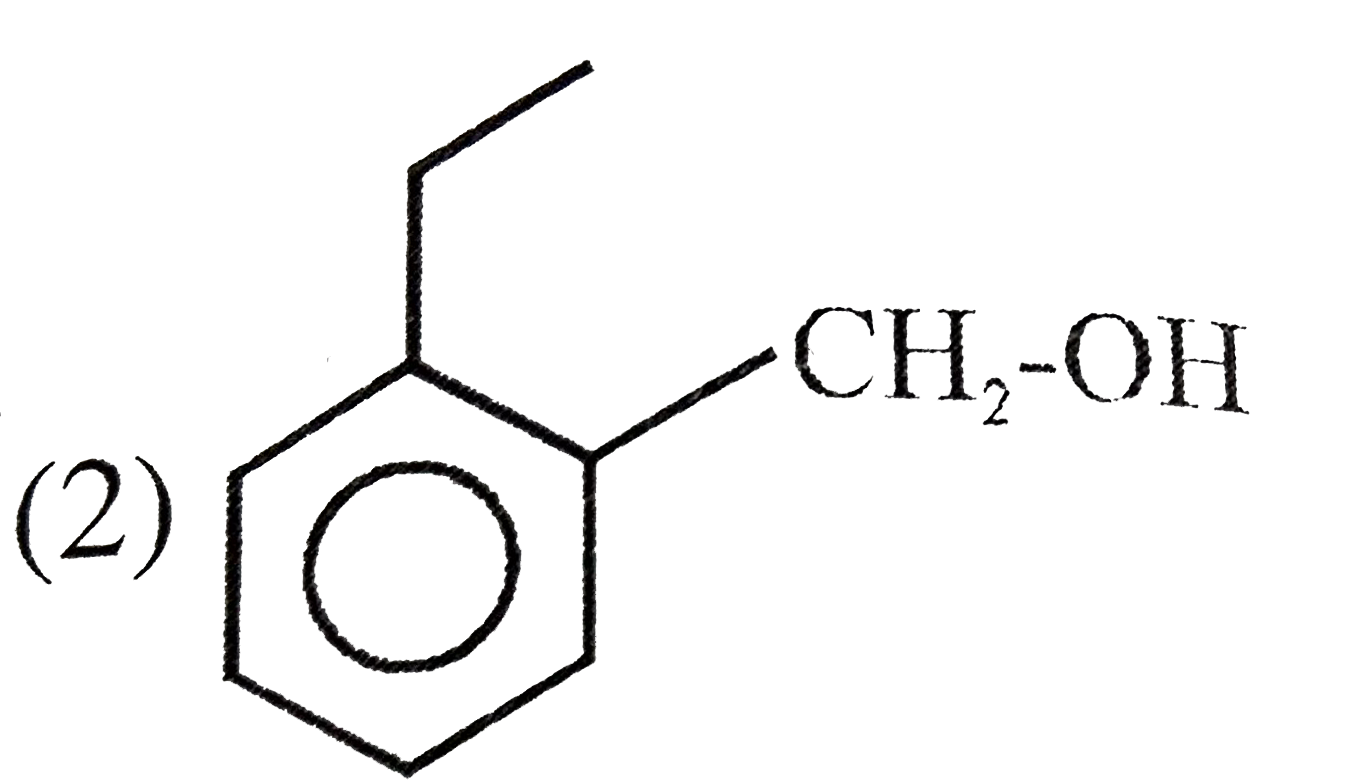
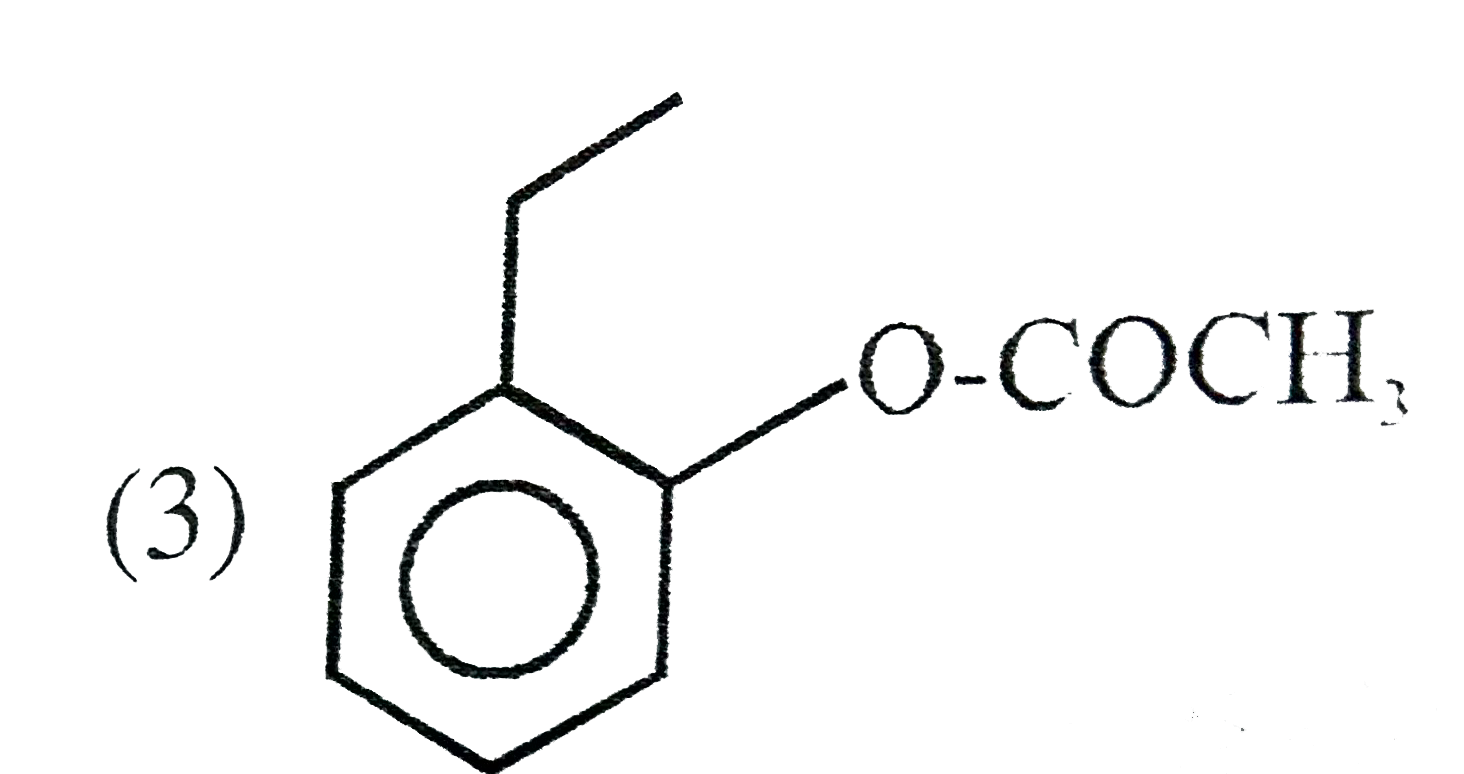
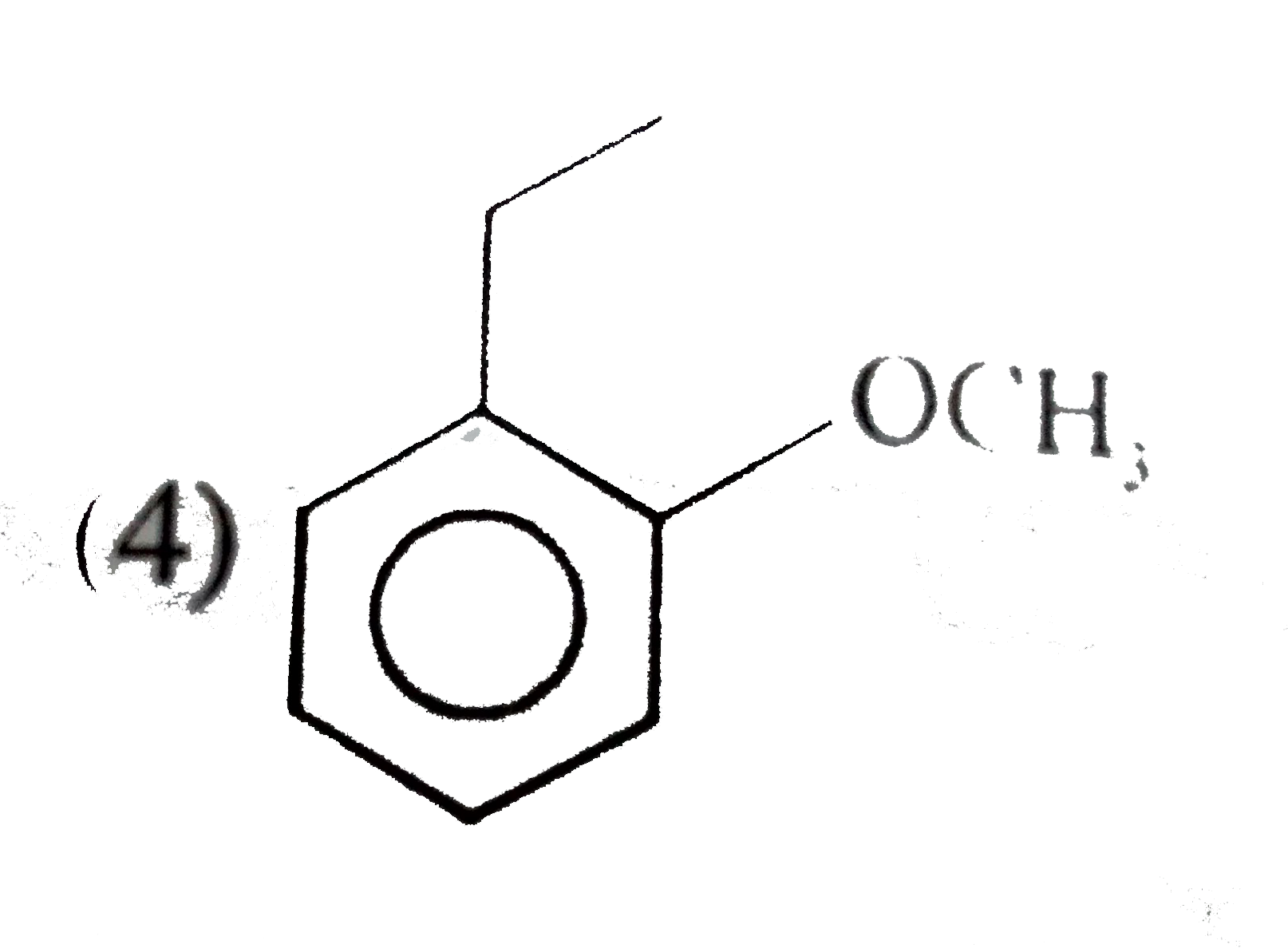
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- The correct Increasing order of Reactivity for following molecule towa...
Text Solution
|
- Nitrobenzene can be prepared from benzene by using a mixture of conc....
Text Solution
|
- Which one the most reactive towards electrophilic reagent:
Text Solution
|
- Which of the following compounds will not undergo Friedal-Craft reacti...
Text Solution
|
- Correct Reactivity order of E.S.R :
Text Solution
|
- Most reactive towords E.S,R :
Text Solution
|
- Correct Decreasing order of pK(b) value of Following Compound is
Text Solution
|
- In which of the following C-Cl Bond ionisation shall give most stable ...
Text Solution
|
- Which of the following is most basic ?
Text Solution
|
- Which one of the following is most stable
Text Solution
|
- Which of the following is insoluble in NaHCO(3) ?
Text Solution
|
- Which is the correct decreasing order of basic strength in aqueous med...
Text Solution
|
- Which of the following is correct order of acidic strength
Text Solution
|
- The correct order of increasing basic nature for the bases NH3....
Text Solution
|
- Which carbocation is most stable
Text Solution
|
- Which of the following group has maximum hyper conjugation effect but ...
Text Solution
|
- What is the correct order of decreasing stability of the following cat...
Text Solution
|
- Phenol and carboxylic acid can be distinguished by
Text Solution
|
- Arrange the following in order of their leaving group tendency ? un...
Text Solution
|
- Arrange the following in order of their decreasing acidic strength ?
Text Solution
|