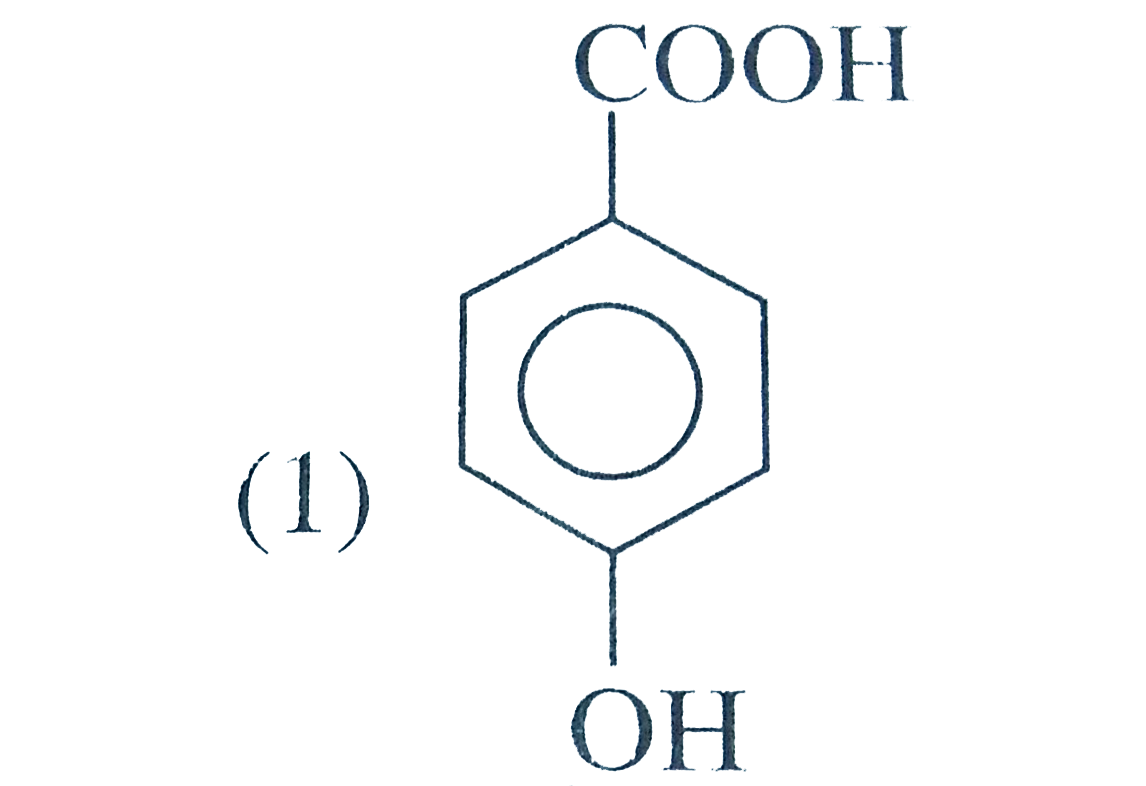
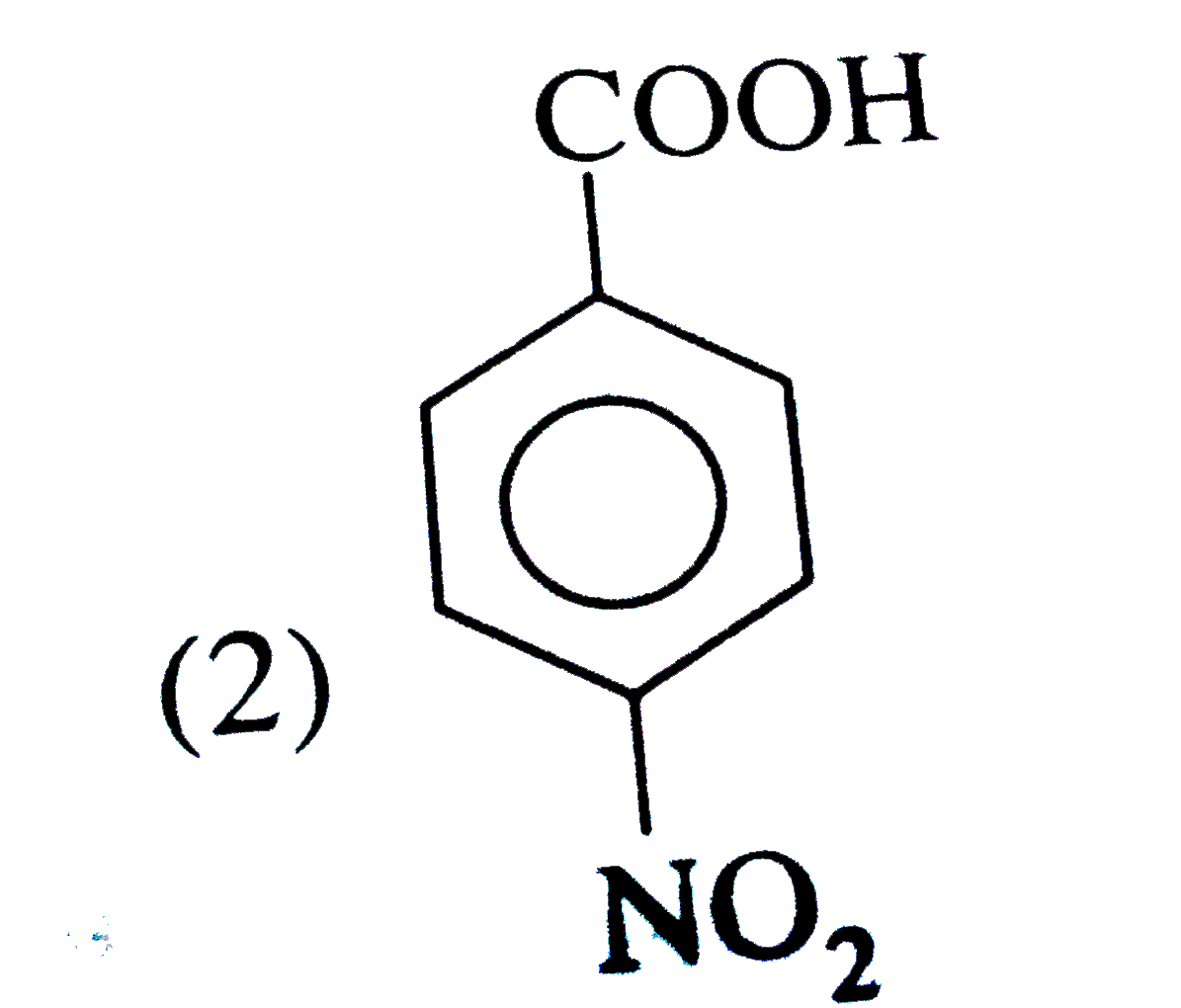
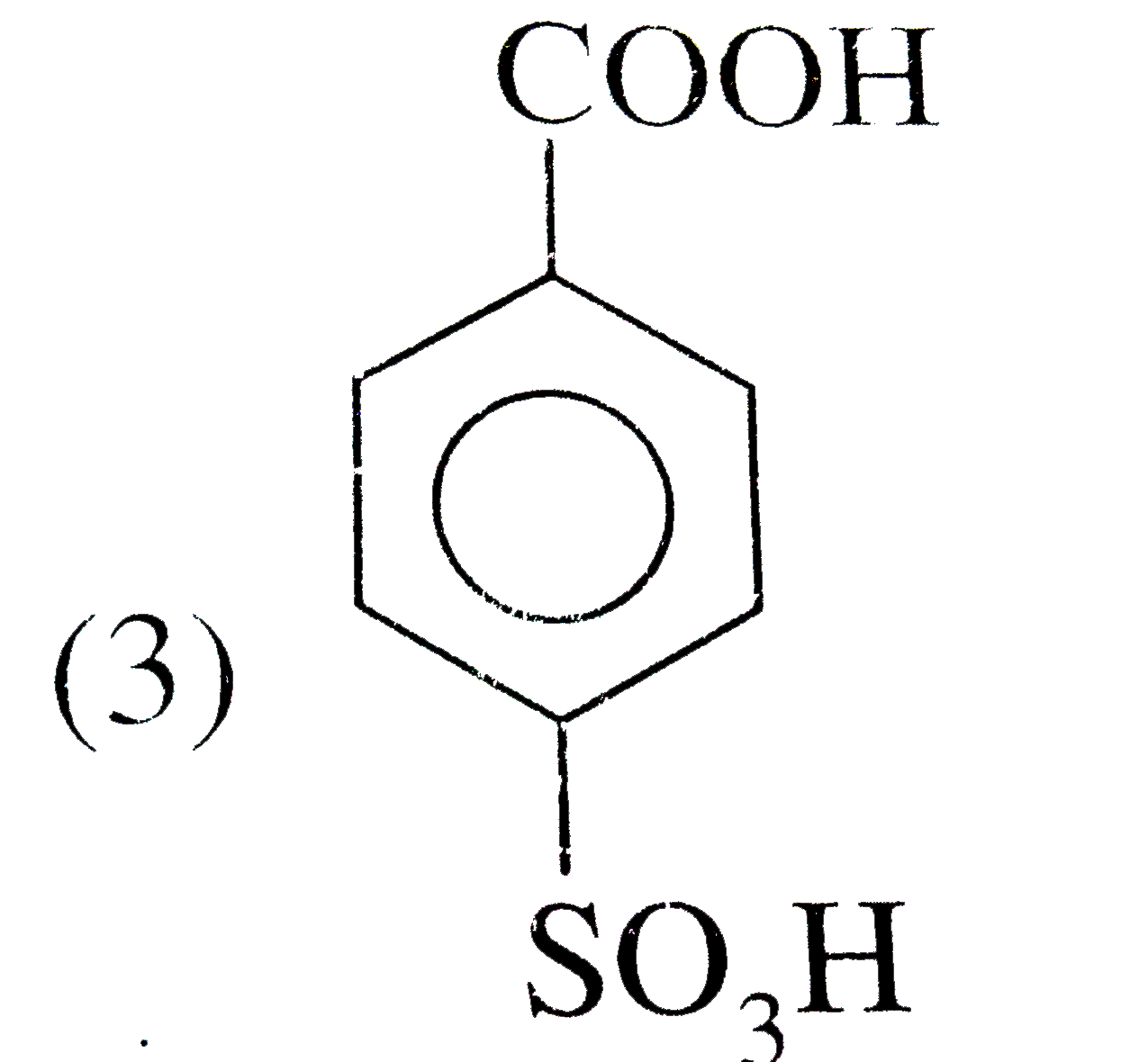
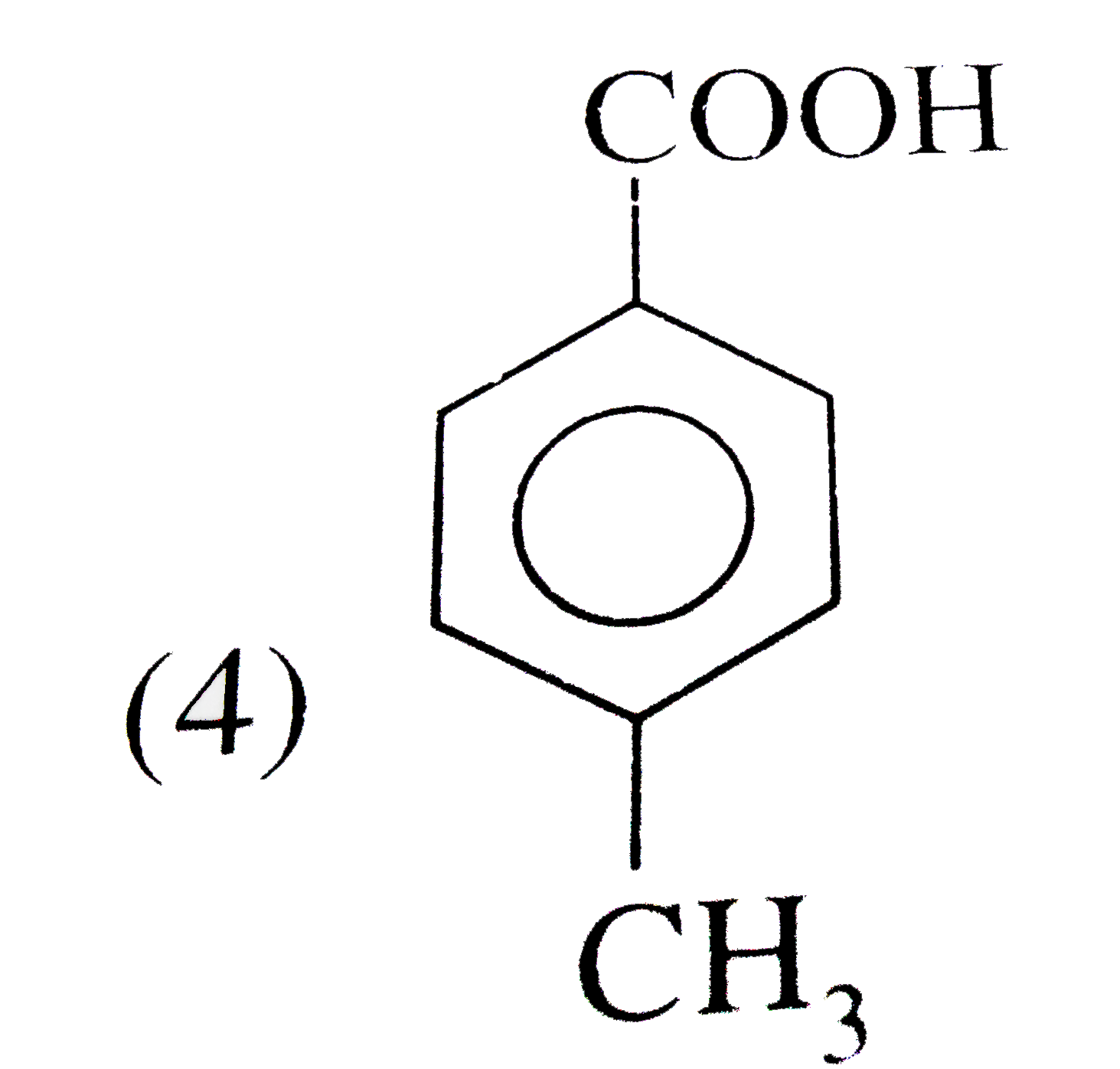A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- Arrange the following in order of their reactivity towords nitration
Text Solution
|
- (oversetE^(+))to major product major product will be
Text Solution
|
- Which of the following is strongest acid among following
Text Solution
|
- . Which of the following is not an electrophile?
Text Solution
|
- The most unlikely resonating structures of pnitrophenoxide ion is :
Text Solution
|
- Which of the following carbanion is most stable ?
Text Solution
|
- Polarisation of sigma - bond is caused by polarisation of adjacent sig...
Text Solution
|
- Select the molecule with more polar bond from each of the indicated bo...
Text Solution
|
- Which of the following does not represent a correct set of nucleophile...
Text Solution
|
- Which of the following is correct regarding formation of reactive inte...
Text Solution
|
- In nitromethane two N-O bond lengths are :
Text Solution
|
- Correct order of stability of the following resonating structure is ...
Text Solution
|
- Which of the following have +M effect (overline e - donating mesomer...
Text Solution
|
- Which of the following compounds does not show electromeric effect
Text Solution
|
- Which of the following is not an example of +E effect:
Text Solution
|
- Cyclooctatetraene is not aromatic . The most important reason for this...
Text Solution
|
- Phenyl magnesium bromide reacts with methanol to give
Text Solution
|
- Identify the end product in the given reaction sequence
Text Solution
|
- The compound formed as a result of oxidation of ethyl benzene by KMnO(...
Text Solution
|
- The main product of the following reaction is C(6)H(5)CH(2)(OH)CH(CH(3...
Text Solution
|