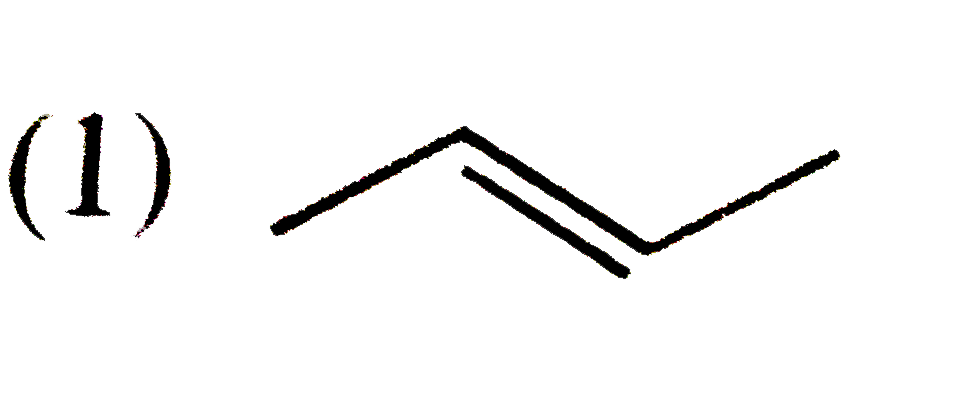
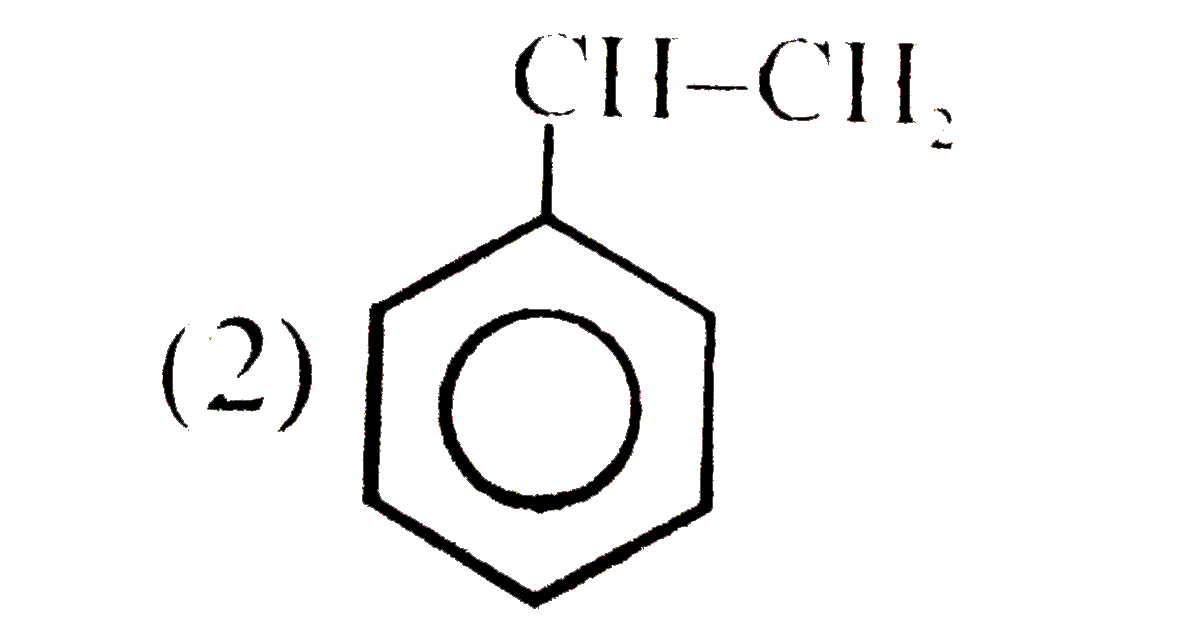
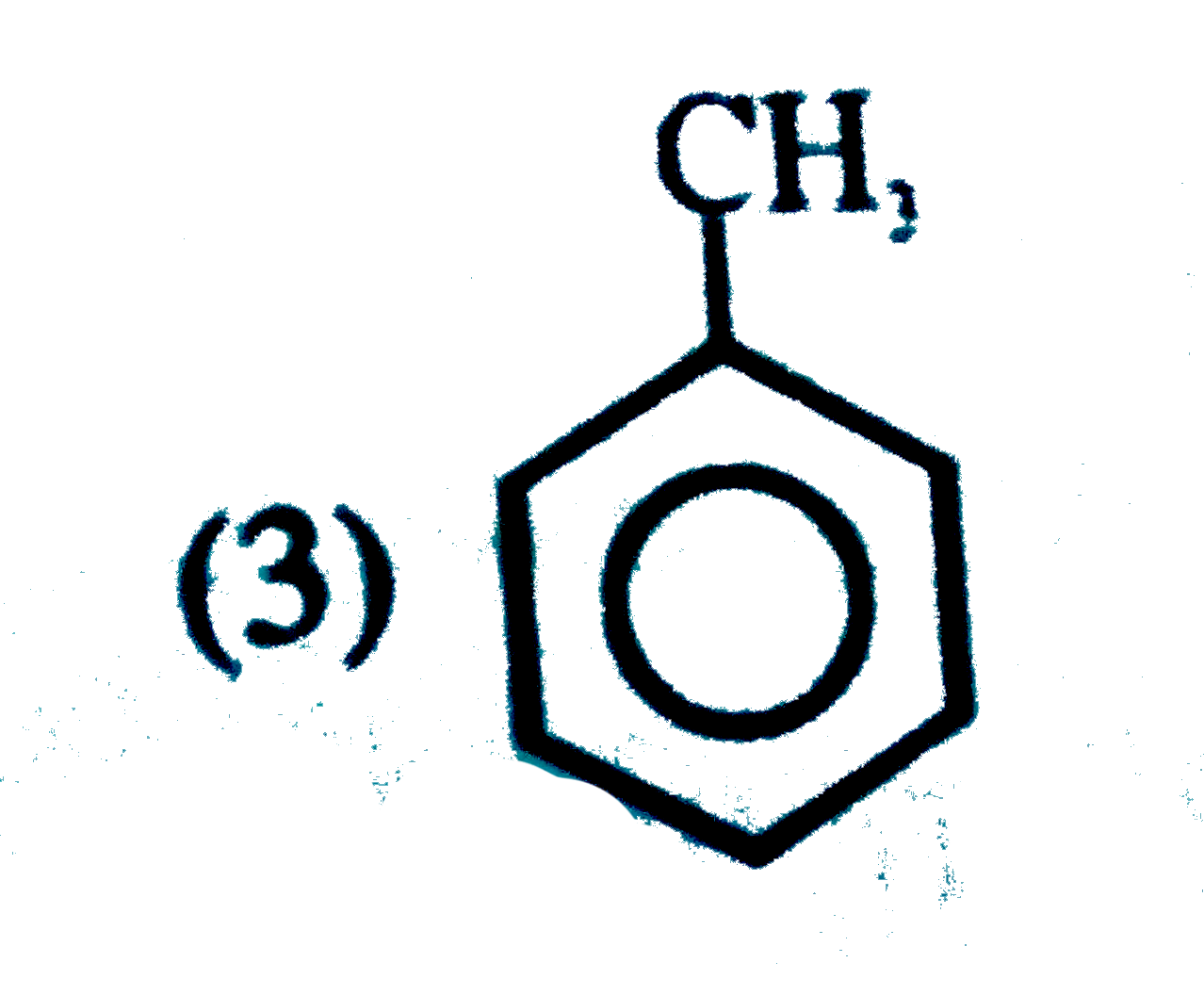
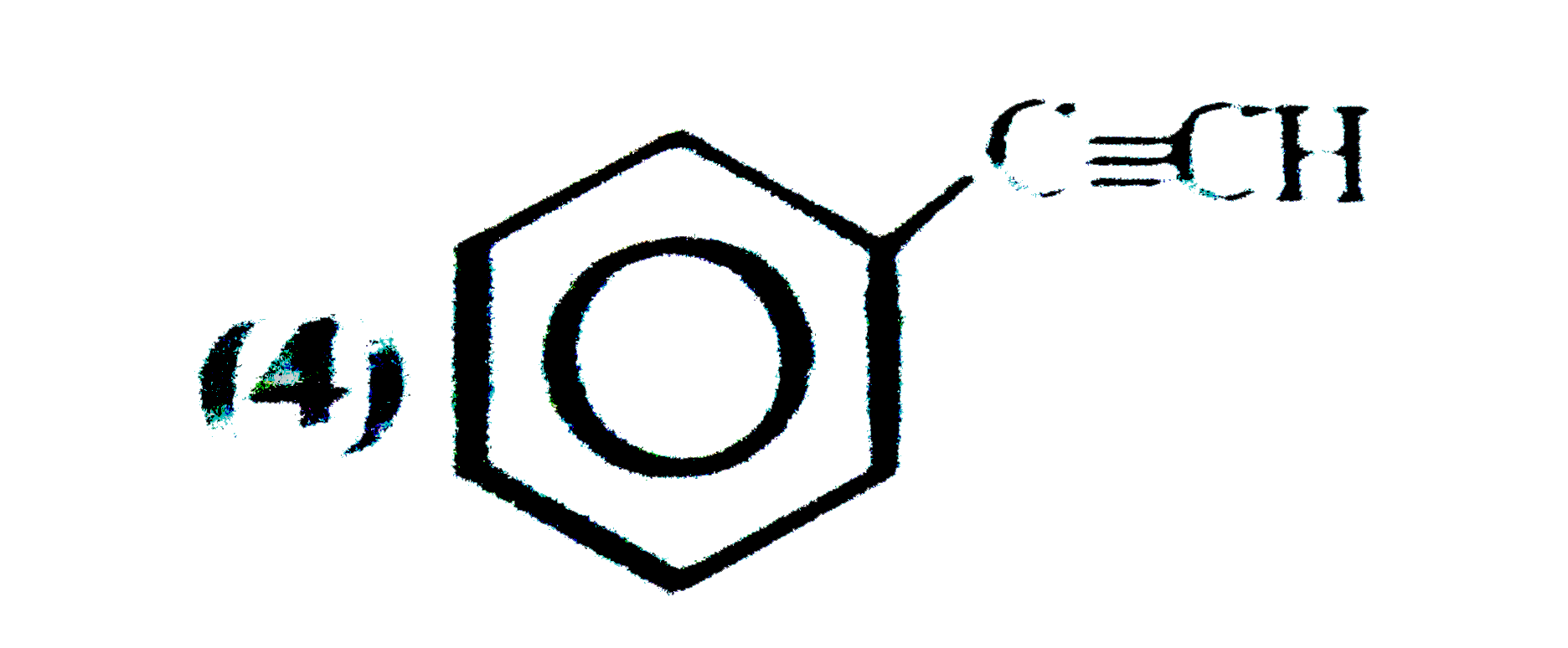
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- The main product of the following reaction is C(6)H(5)CH(2)(OH)CH(CH(3...
Text Solution
|
- CH(3)-underset(Cl)underset(|)(C)H-underset(Cl)underset(|)(C)H-CH(3)und...
Text Solution
|
- Which of the following does not decolourise reddish brown solution of ...
Text Solution
|
- Reagent x can be
Text Solution
|
- Which one of the following compounds will give white precipitate wit...
Text Solution
|
- Acetylene on heating in the presence of red hot Fe tube gives :
Text Solution
|
- Which among the following alkenes will be most stable :
Text Solution
|
- Which among the following compounds will give Wurtz reaction ?
Text Solution
|
- In the following reaction : C(2)H(2)underset(HgSO(4)//H(2)SO(4))ove...
Text Solution
|
- One of the following which does not observe the anti-Markovnikoff's ad...
Text Solution
|
- Propyne and propene can be distinguished by conc. H2SO4 Br2 in "CCl"...
Text Solution
|
- which of these will not react with acetylene ?
Text Solution
|
- What happens when acetylene is treated with hypochlorous acid
Text Solution
|
- Complete the following reaction
Text Solution
|
- CH(3)-underset(CH(3))underset(|)overset(CH(3))overset(|)C-CH=CH-CH(3)o...
Text Solution
|
- During debromination of meso-dibromobutane, the major compound formed ...
Text Solution
|
- Which is maximum reactive towords acid catalysed dehydration :
Text Solution
|
- Which gives ketonic group after hydroboration oxidation :
Text Solution
|
- Complete the following reaction
Text Solution
|
- on oxymercuration demercuaration produces the major product
Text Solution
|