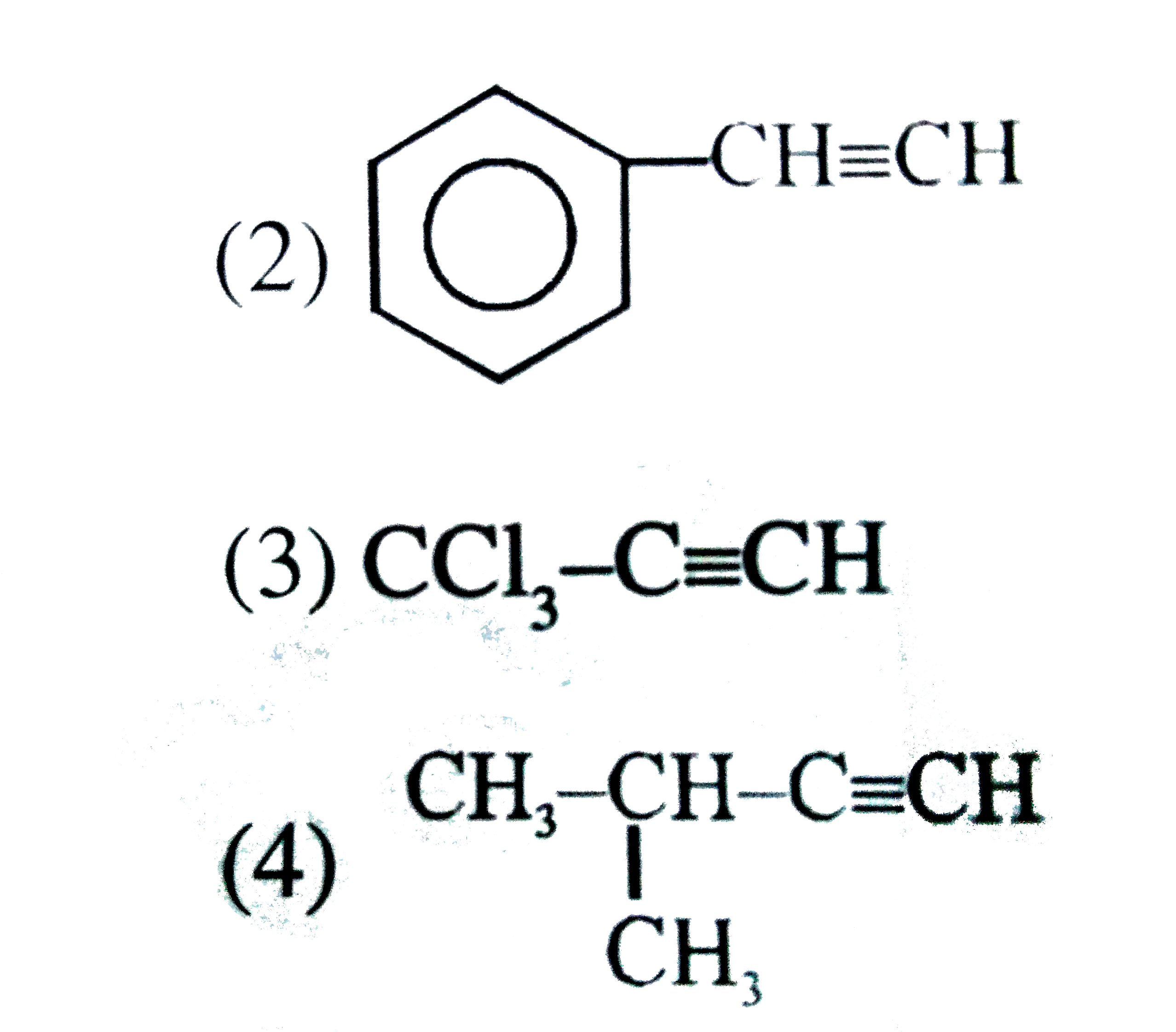
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- During debromination of meso-dibromobutane, the major compound formed ...
Text Solution
|
- Which is maximum reactive towords acid catalysed dehydration :
Text Solution
|
- Which gives ketonic group after hydroboration oxidation :
Text Solution
|
- Complete the following reaction
Text Solution
|
- on oxymercuration demercuaration produces the major product
Text Solution
|
- How many chiral compounds are possible on monochlorination of 2-methyl...
Text Solution
|
- Ozonolysis of an organic compound A produces acetone and propionaldehy...
Text Solution
|
- Arrange the following in decreasing order of reactivity towards electr...
Text Solution
|
- Which of the following is most basic
Text Solution
|
- Ph-CH=CH-Ph underset(C Cl(4))overset(Cl(2))rarr X overset(2NaNH(2))rar...
Text Solution
|
- Which Intermediate formed during addition of halogen on alkenes ?
Text Solution
|
- CH(3)-C-=CHunderset(dil.H(2)SO(4))overset(HgSO(4))(to) product Produ...
Text Solution
|
- Product, Product is
Text Solution
|
- No. of structural isomeric alkenes (molecular formula =C(6)H(12)) whic...
Text Solution
|
- Arrange the following towards their reactivity for electrophilic addit...
Text Solution
|
- Arrange the following towards reactivity for heat of combustion :-
Text Solution
|
- Benzene + Acetylchloride overset(AlCl(3))(to) product Name of the abov...
Text Solution
|
- Which of the following Alkane can not be prepared by hydrogenation o...
Text Solution
|
- From how many alkyl halide we get pentane on reaction with Zn + HCl ...
Text Solution
|
- C(2)H(5)Br+CH(3)-underset(CH(3))underset(|)(CH)-Broverset((Na)dry ethe...
Text Solution
|