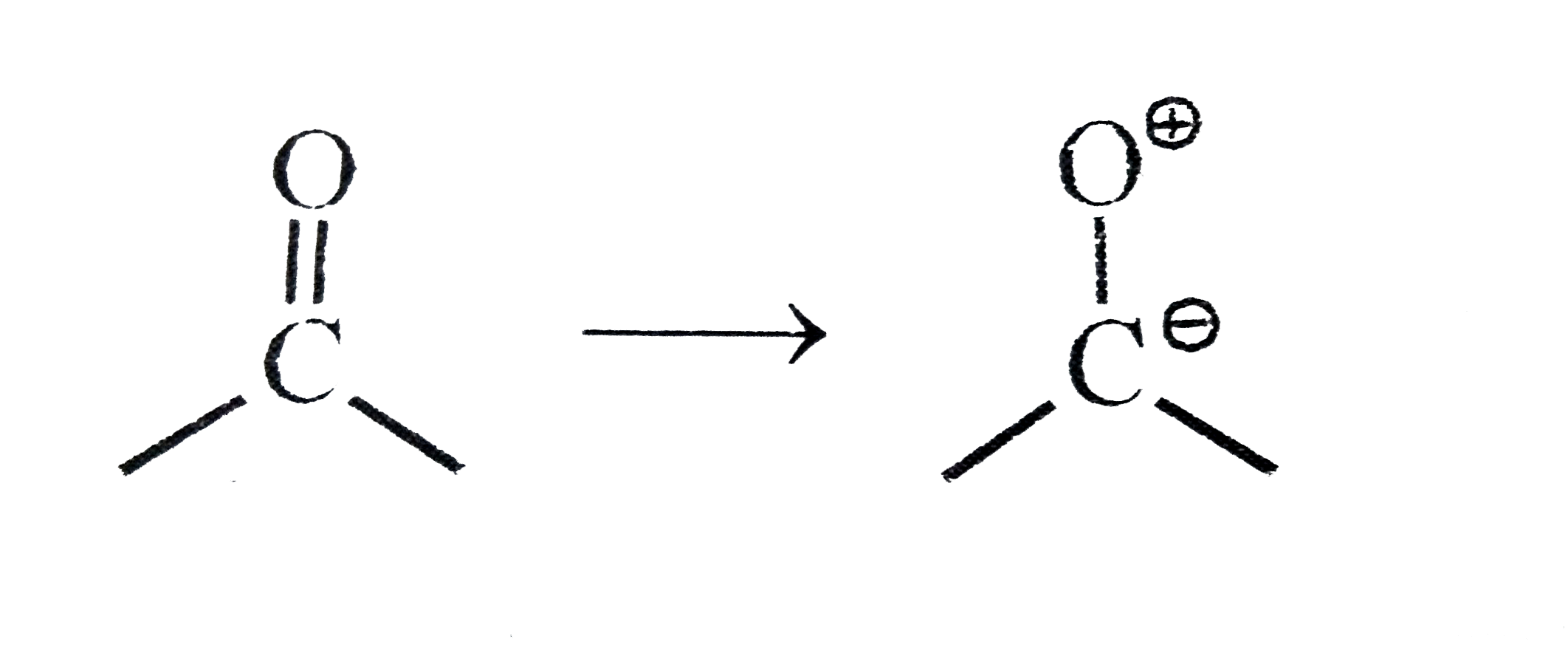
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- (a) Phthalaldehyde overset(Conc.NaOH)(to) Product (to) Cannizzaro rea...
Text Solution
|
- Which of the following is correct reaction : (P) Oxalic acid overset...
Text Solution
|
- Which of the following statement is not true about carbonyl group :
Text Solution
|
- Which of the following reaction from benzene carbaldehyde as a product...
Text Solution
|
- C is :
Text Solution
|
- Which of the following is not correct matched :
Text Solution
|
- Which of the following product is not correct : Carbonyl compound + ...
Text Solution
|
- Which of the compound have most acidic alphaH:
Text Solution
|
- Benzoic acid not form in :
Text Solution
|
- C is :
Text Solution
|
- How many compounds gives Cannizzaro reaction and aldol condensation re...
Text Solution
|
- X is :
Text Solution
|
- Which of the following set is correct : Compound Positive test of ...
Text Solution
|
- Which of the following compounds not shows positive Tollen's Test
Text Solution
|
- Acetophenone not produced in which reaction :
Text Solution
|
- Which reaction shows decrease in number of carbon atoms in product :
Text Solution
|
- Z may not be:
Text Solution
|
- Aoverset(H(3)O^(+))(to)B+EtOH Boverset(Delta)(to) Acetone A is :
Text Solution
|
- CH(3)-C-=Nunderset(2.H(3)O^(+))overset(1.SnCl(2)//HCl)(to) Product R...
Text Solution
|
- Correct stament for nucleophlic addition of sodium bisulphate on carbo...
Text Solution
|