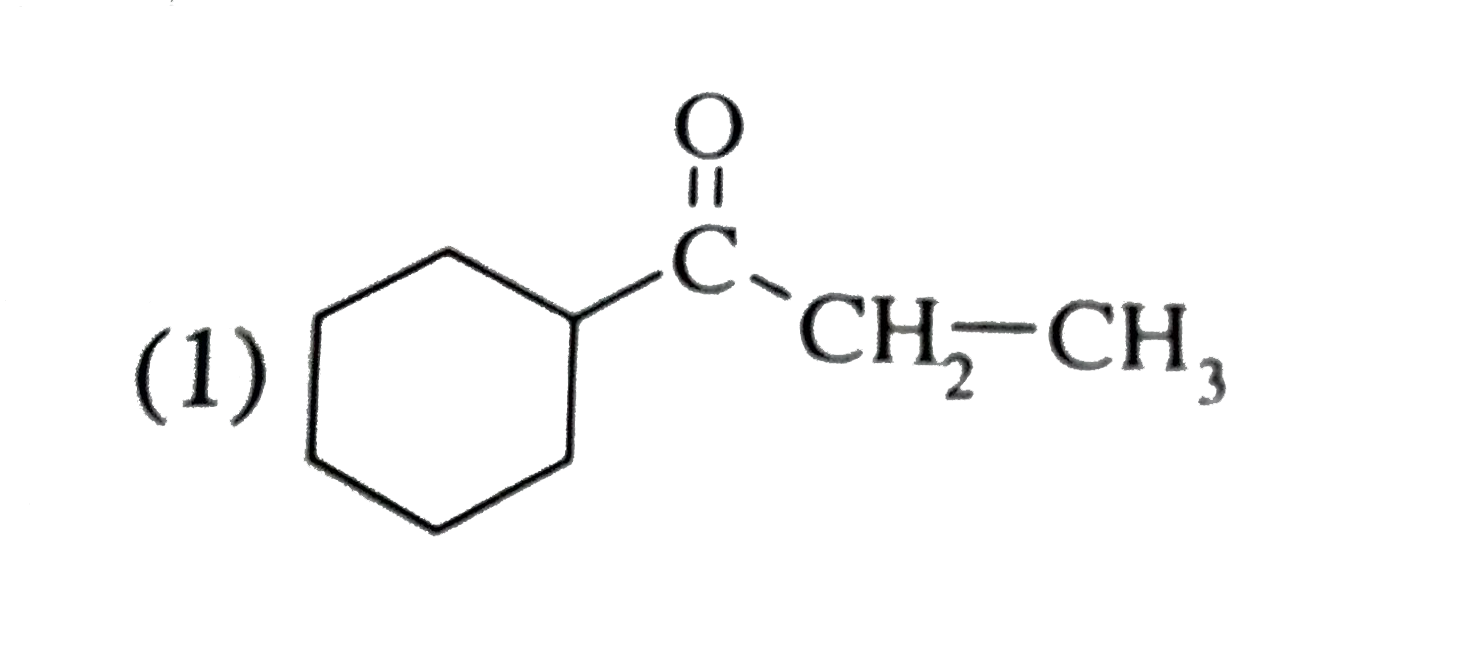
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- Complete the following reaction
Text Solution
|
- Correct order of dehydraton of aldol:
Text Solution
|
- Which of the following compound can from hydrazone and will give iodof...
Text Solution
|
- Propanal on treatment with dilute sodium hydroxide gives
Text Solution
|
- Which of the following reactions will not result in the formation of c...
Text Solution
|
- Which of the following compounds is most susceptible to a nucleophilic...
Text Solution
|
- In a set of reactions propionic acid yielded a compound D. CH3CH2COO...
Text Solution
|
- Acetaldehyde and benzaldehyde can not be distinguished by
Text Solution
|
- Which of the following compounds is/not oxidised by Fehling's solution...
Text Solution
|
- RCH(2)OHoverset([O])(to)RCHO Which of the following oxidising agent...
Text Solution
|
- CH(3)-C-=CHunderset(Hg^(+2))overset(dil.H(2)SO(4))(to)Xunderset(Delta)...
Text Solution
|
- Which of the following is correct for
Text Solution
|
- Acetaldehyde and benzaldehyde can not be distinguished by
Text Solution
|
- The above reaction is known as
Text Solution
|
- Which of the following reaction is not correct according to major prod...
Text Solution
|
- Product B is
Text Solution
|
- In which reaction racemic mixture is obtained as product ?
Text Solution
|
- Arrange the following in order of their reactivity towards CH(3)MgBr? ...
Text Solution
|
- major product
Text Solution
|
- Which of the following do not give aldol reaction with dil. NaOH
Text Solution
|