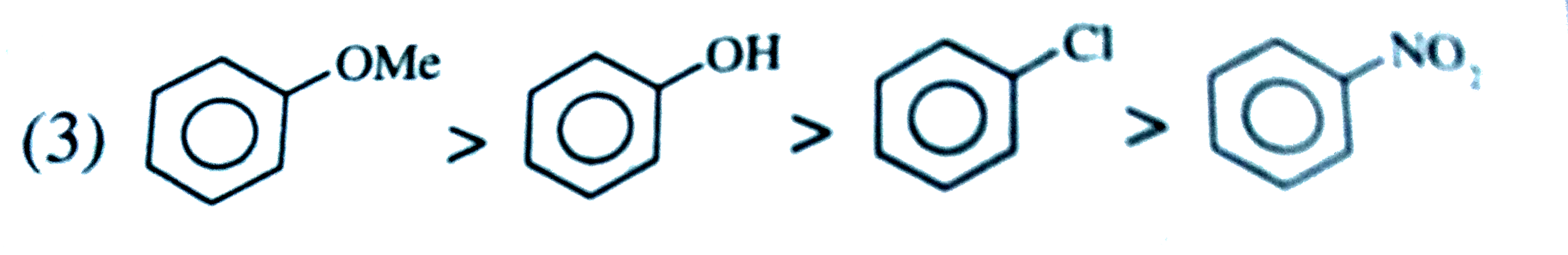A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- RCH(2)OHoverset([O])(to)RCHO Which of the following oxidising agent...
Text Solution
|
- CH(3)-C-=CHunderset(Hg^(+2))overset(dil.H(2)SO(4))(to)Xunderset(Delta)...
Text Solution
|
- Which of the following is correct for
Text Solution
|
- Acetaldehyde and benzaldehyde can not be distinguished by
Text Solution
|
- The above reaction is known as
Text Solution
|
- Which of the following reaction is not correct according to major prod...
Text Solution
|
- Product B is
Text Solution
|
- In which reaction racemic mixture is obtained as product ?
Text Solution
|
- Arrange the following in order of their reactivity towards CH(3)MgBr? ...
Text Solution
|
- major product
Text Solution
|
- Which of the following do not give aldol reaction with dil. NaOH
Text Solution
|
- How many aldol product will be obtained in the following reaction CH...
Text Solution
|
- which of the following contain most acidic hydrogen
Text Solution
|
- (a) write the products formed when CH(3)CHO reacts with the following ...
Text Solution
|
- Which gives aldehyde on hydration by H(2)SO(4) and HgSO(4)
Text Solution
|
- ?underset(2.H(2)O)overset(1.Delta//DIBAL-H)(to)CH(3)-CH=CH-CH(2)-CH(2)...
Text Solution
|
- CH(3)-underset(O)underset(||)(C)-CH(3)+underset(("semicarbazide"))(NH(...
Text Solution
|
- Ph-underset(O)underset(||)(C)-CH(3)overset(NaOCl)(to)A+ "salt" A+CH(...
Text Solution
|
- Complete the following reaction
Text Solution
|
- CH(3)-CH=CH-underset(O)underset(||)(C)-CH(3)+NH(2)-OHoverset(H^(o+))(t...
Text Solution
|