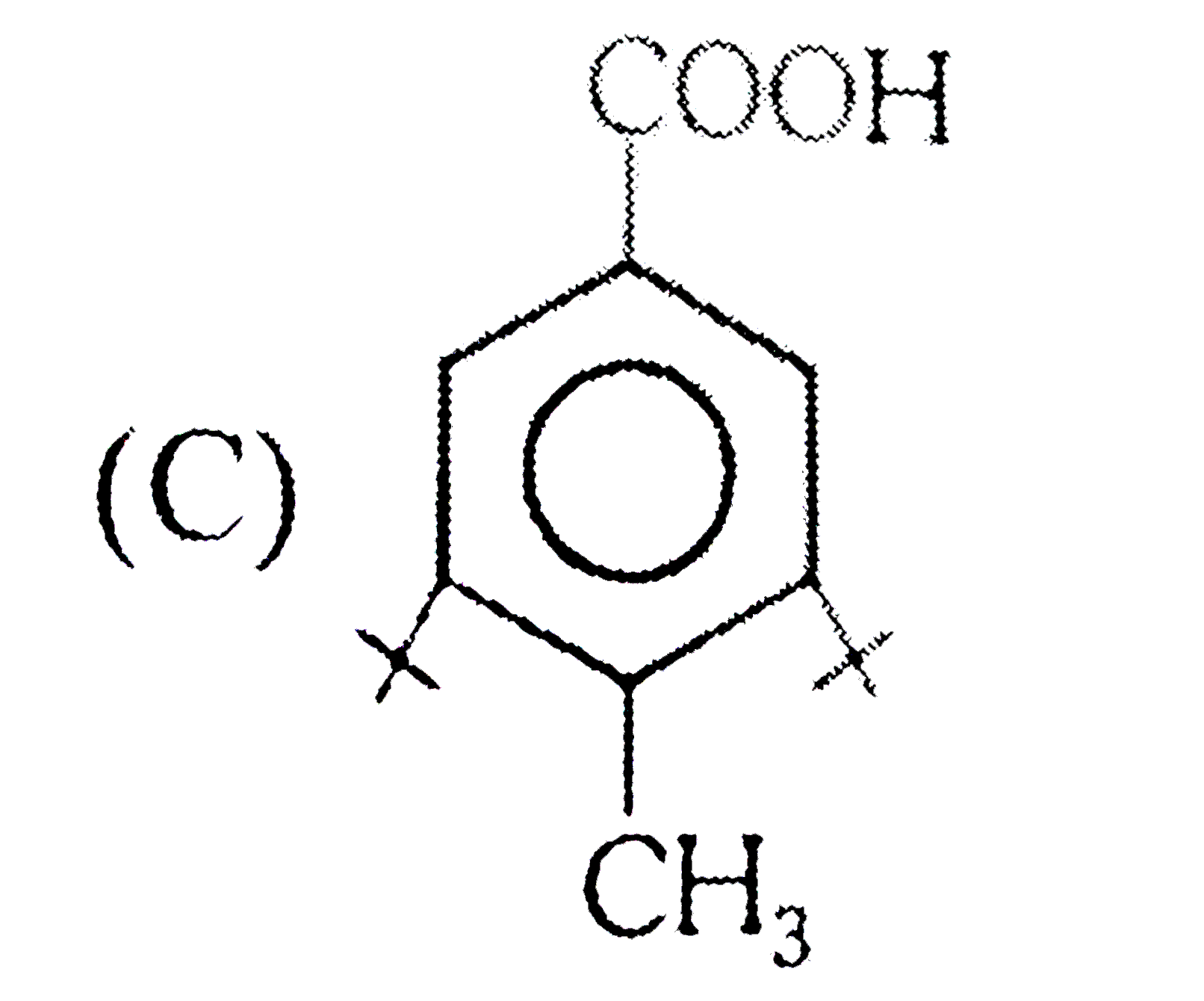
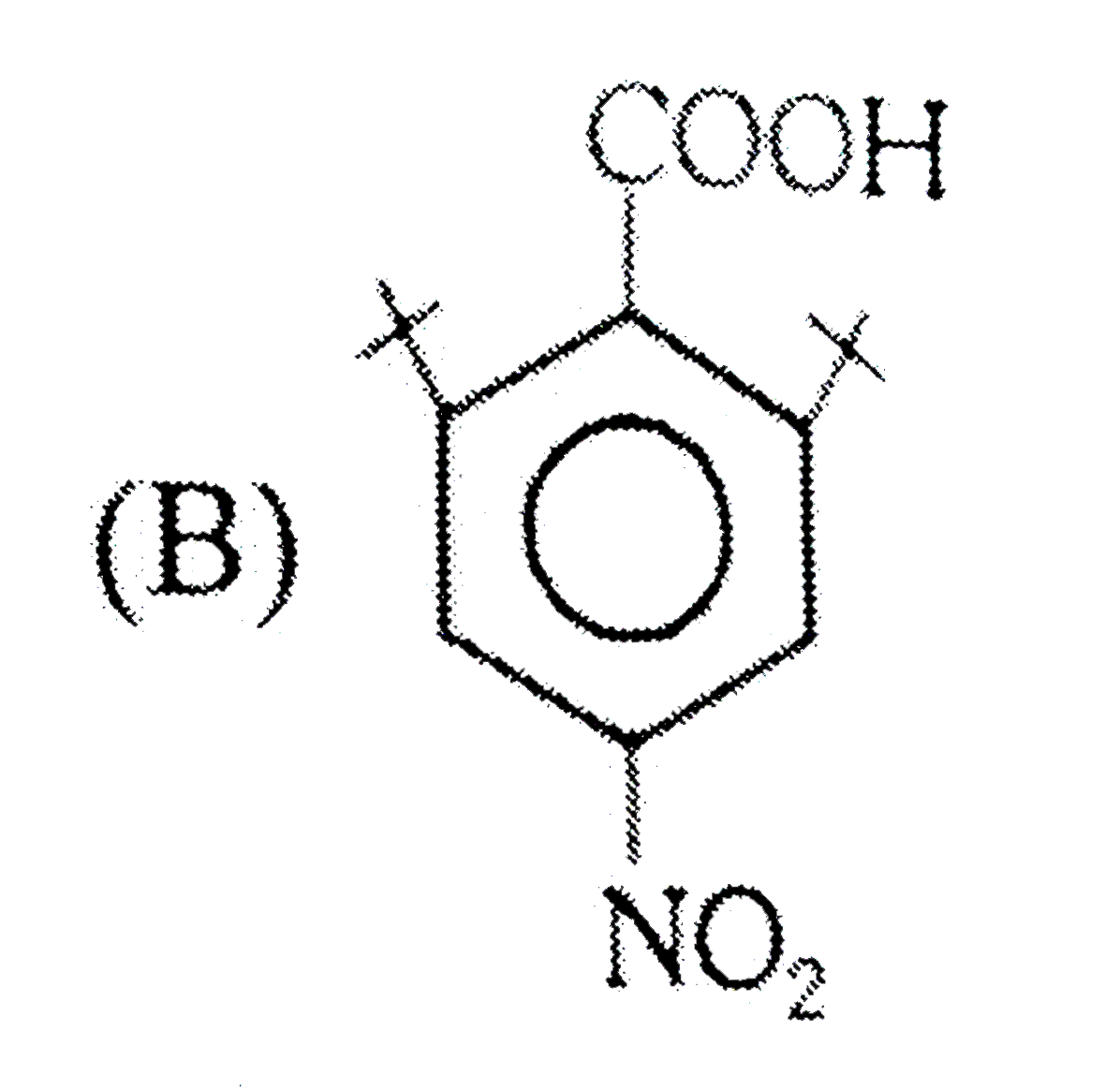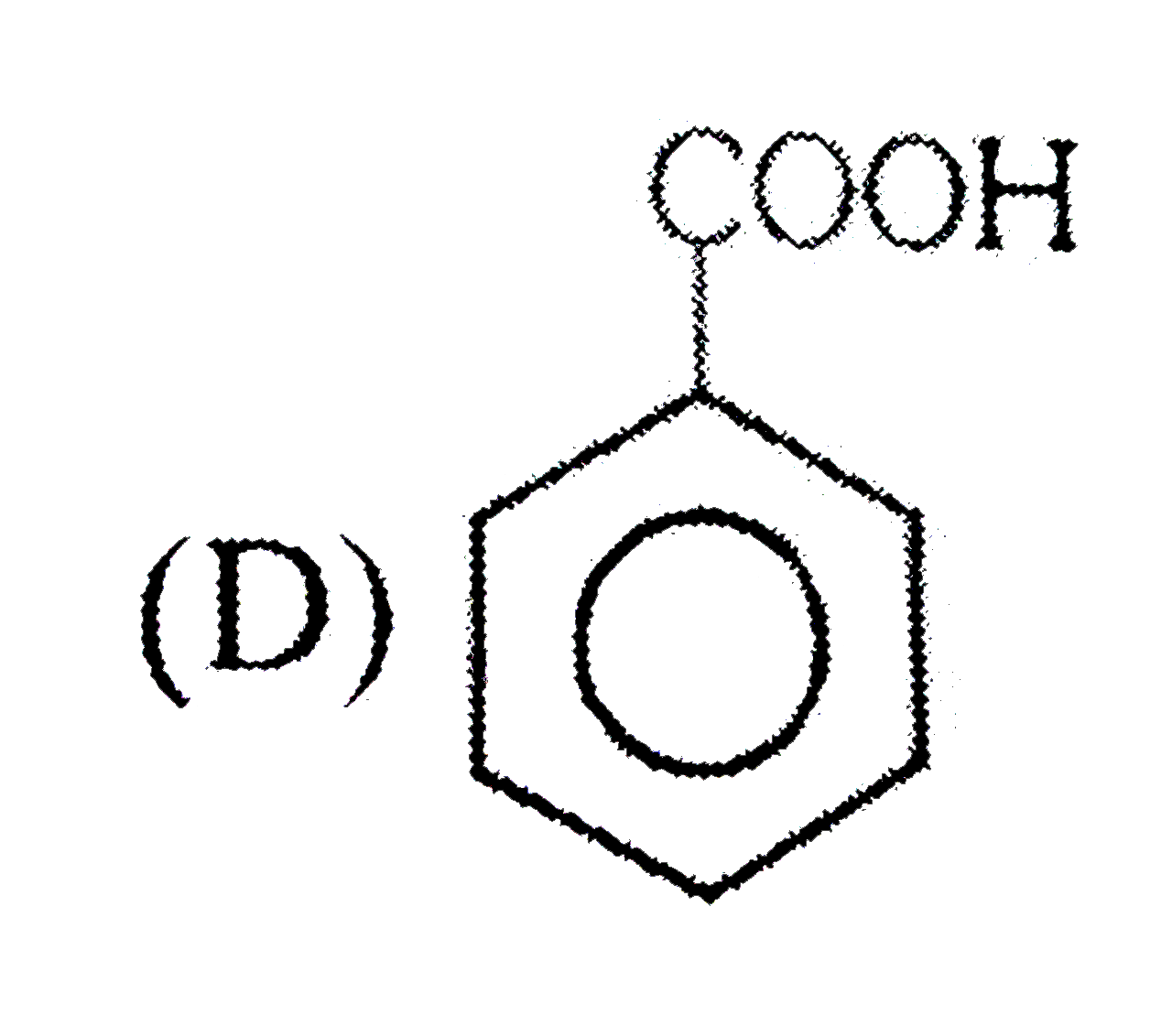A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ACIDIC STRENGTH & BASIC STRENGTH-Exercise V
- The most important condition for resonanace to occur is that the invov...
Text Solution
|
- In the following compounds The order of acidity is
Text Solution
|
- Although phenoxide ion has more number of resonating structures than c...
Text Solution
|
- Amongst the following, the most basic compound is-
Text Solution
|
- The correct order of basicities of the following compounds is , " ...
Text Solution
|
- Statement-1:p-Hydroxybenzoic acid has a lower boiling point that o-Hyd...
Text Solution
|
- Match K(a) values with suitable acid:
Text Solution
|
- Which of the following is more acidic and why?
Text Solution
|
- The product A will be
Text Solution
|
- Correct order of acidic strength among the following oxides is:
Text Solution
|
- Amongst the following, the number of compounds soluble in aqueous NaOH...
Text Solution
|
- Among the following compounds the most acidic is
Text Solution
|
- The carboxyl functional group(-COOH) is present in:
Text Solution
|
- Identify the binary mixtrues (s) that can be separated nto the individ...
Text Solution
|
- The compound that does not liberate CO2 on treatment with aqueous sod...
Text Solution
|
- Hydrogen bonding plays a central role in the following phenomena .
Text Solution
|
- The increasing order of basicity of the following compounds is
Text Solution
|