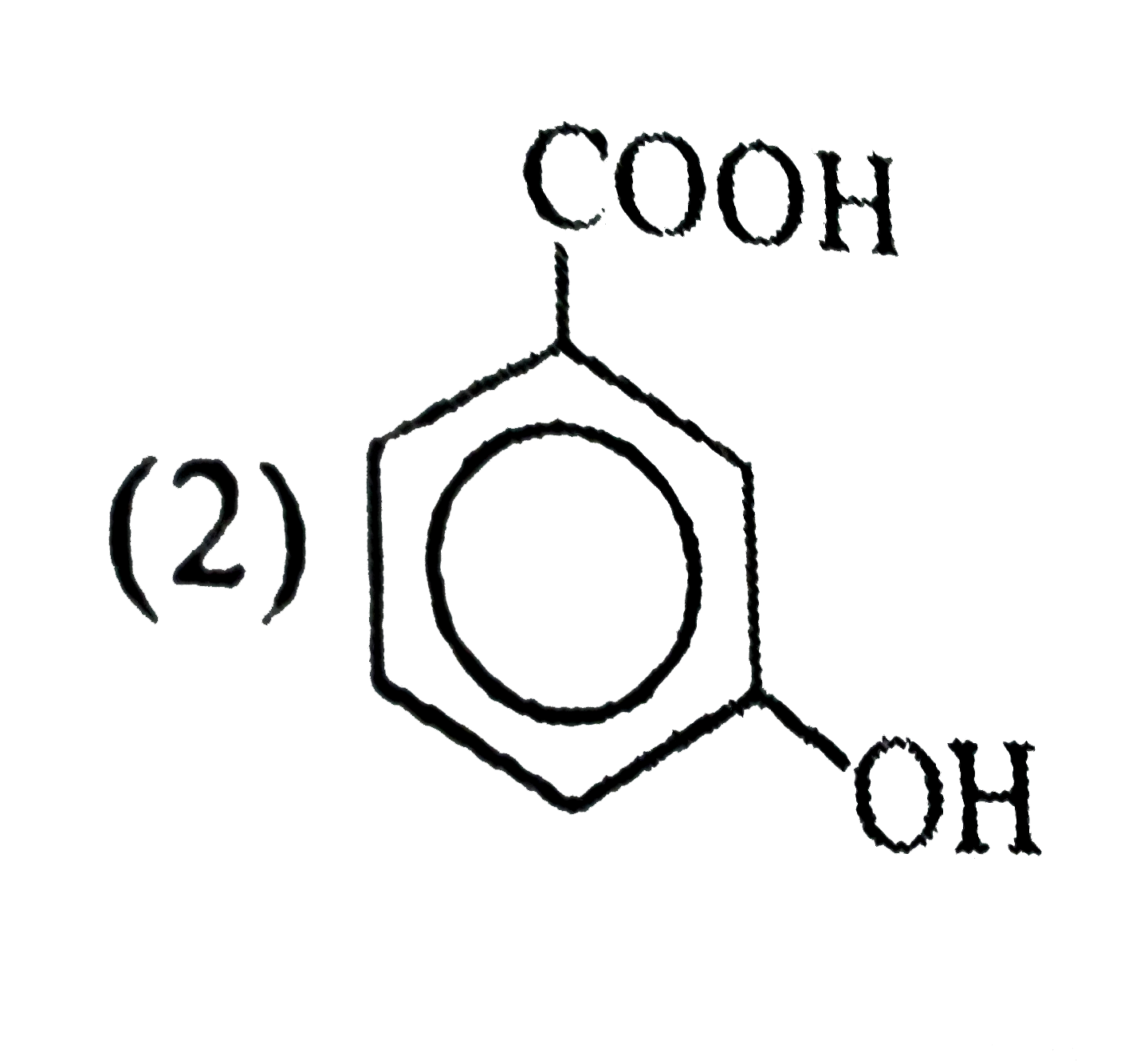
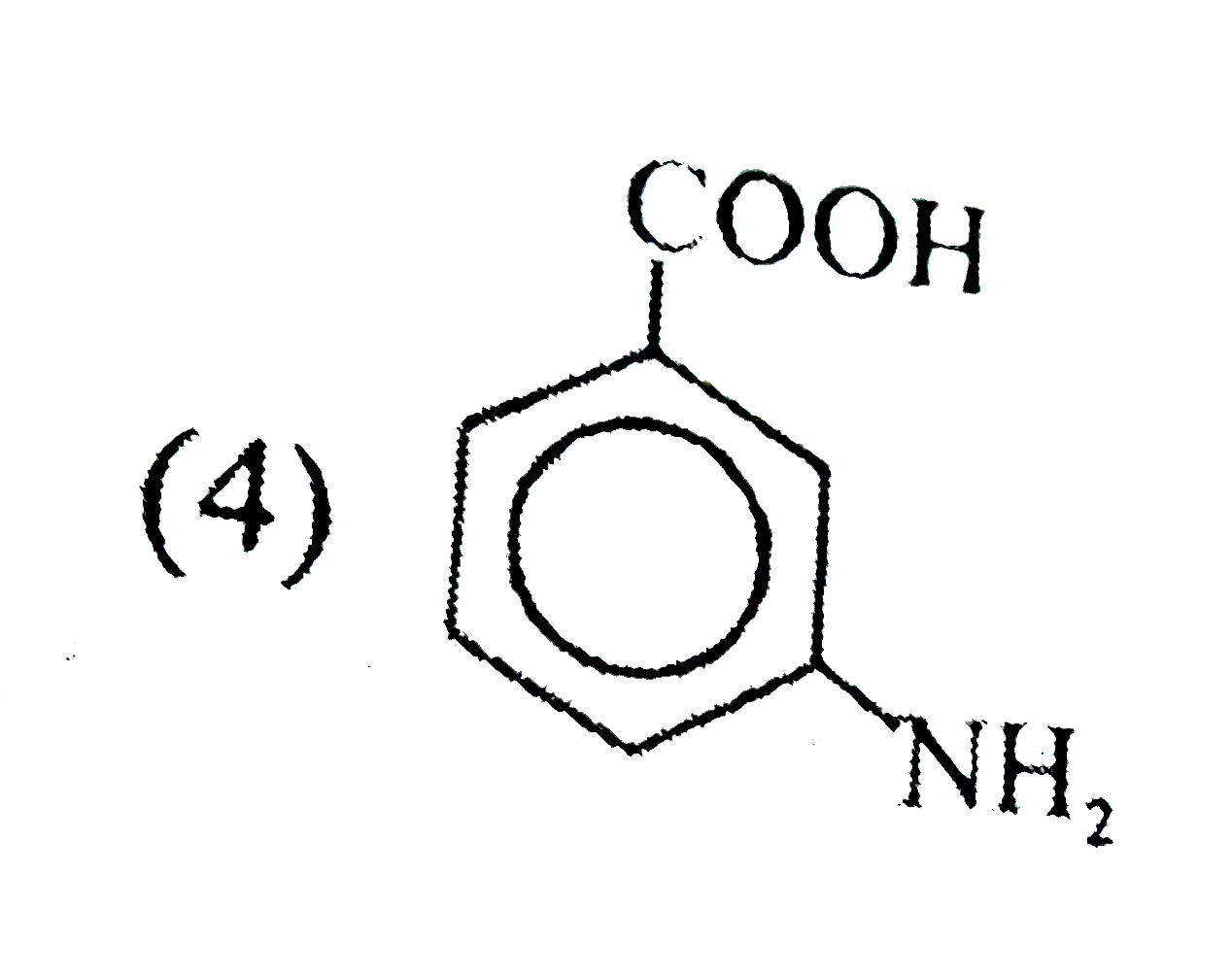A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ACIDIC STRENGTH & BASIC STRENGTH-Exercise IV
- Write the structural formulae of the following compounds : (i) Picri...
Text Solution
|
- Species acting as both Bronsted acid and base is:
Text Solution
|
- The correct order of increasing basic nature for the bases NH3....
Text Solution
|
- Consider the acidity of the carboxylic acids: (1) PhCOOH (2) o-NO(...
Text Solution
|
- Which of the following is the strongest base:
Text Solution
|
- Among the following acids, which has the lowest pK(a) value?
Text Solution
|
- Amongest the following the most basic compounds is-
Text Solution
|
- What is the conjugate base of OH^(-) ?
Text Solution
|
- Among the following acids which has the lowest pk(a) value-
Text Solution
|
- The correct order of increasing acid strength of the compounds is (a...
Text Solution
|
- Which of these is the strongest base in aqueous media?
Text Solution
|
- The correct order of increasing basicity of the given conjugate bases ...
Text Solution
|
- The strongest acid amongst the following compounds is?
Text Solution
|
- Arrange the following compounds in increasing order of acidity and giv...
Text Solution
|
- In the following compounds: the order of basicity is as follows:
Text Solution
|
- The most basic compound among the following is-
Text Solution
|
- Most basic amine in the gas phase is :
Text Solution
|
- Arrange the following compounds in order of decreasing acidity:
Text Solution
|
- Conjugate base of hydrazoic acid HN(3) is :
Text Solution
|
- Which among the following compounds will not give effervescence with s...
Text Solution
|