Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN- CONCENTRATION TERMS-Exercise Jee-Advance
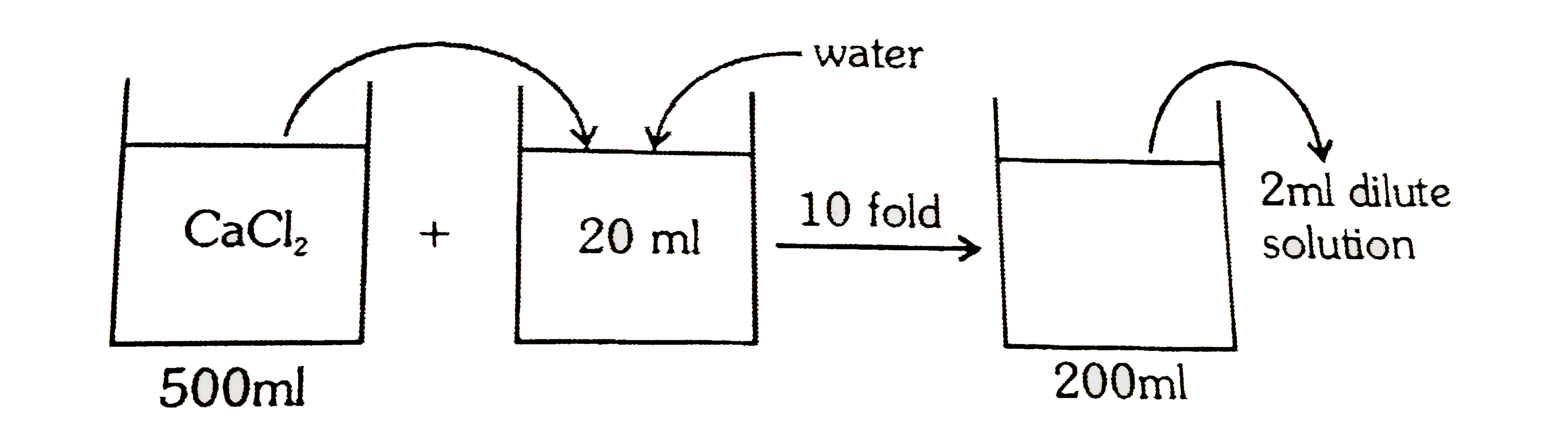
- 1.11g CaCl(2) is added to water forming 500ml of solution 20ml of this...
Text Solution
|
- Calculate the molarity of water if its density is 1000 kg m^(-3)
Text Solution
|
- Dissolving 180 g of glucose (mol.w.t. 180) in 1000g of water gave a so...
Text Solution
|
- A compound H(2)X with molar weight of 80 g is dissolved in solvent ...
Text Solution
|