Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MOLE CONCEPT
ALLEN|Exercise S-I (Problems Related with Different Types of Atomic Masses & Basic Concept of Mole)|52 VideosMOLE CONCEPT
ALLEN|Exercise S-II|18 VideosMOLE CONCEPT
ALLEN|Exercise Some Solved Examples|9 VideosMETALLURGY
ALLEN|Exercise EXERCISE-05 [B]|26 Videosp-Block Element
ALLEN|Exercise All Questions|20 Videos
Similar Questions
Explore conceptually related problems
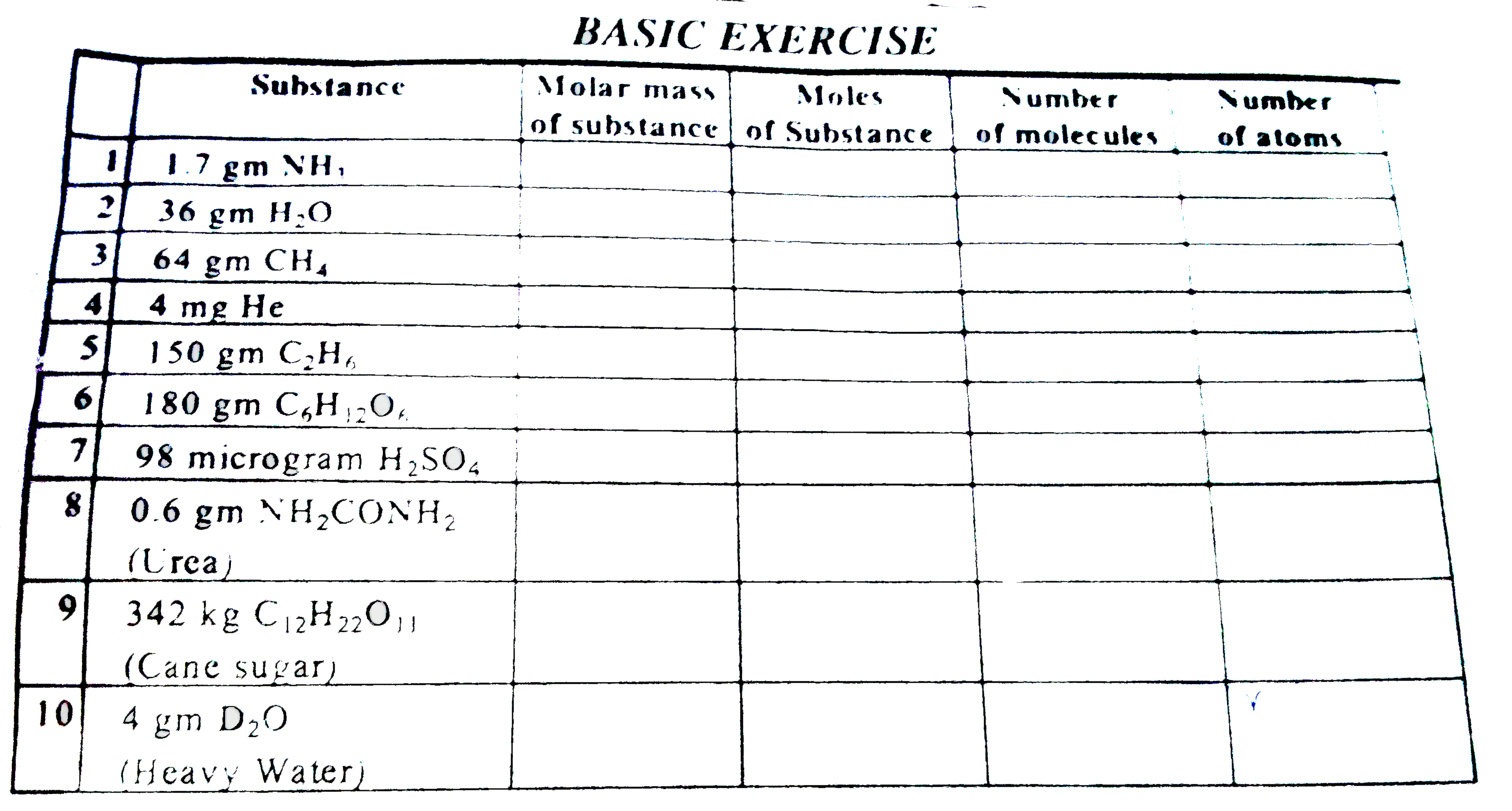
ALLEN-MOLE CONCEPT-Basic Exercise
- Find moles of O-atoms in 5.6 litres of SO(3) at 0^(@)C ,1 atm ?
Text Solution
|
- How many atoms are there in 5 moles of silver (N(A)=6xx10^(23))
Text Solution
|
- How many moles of O-atoms are there in 1 mole CaCO(3)
Text Solution
|
- How many moles of O-atoms are in 2.7xx10^(25) molecules of CO(2) (N(A)...
Text Solution
|
- Find number of O-atoms in 1 moles O(2)
Text Solution
|
- Find moles of Cu atom and number of Cu atoms in it's 0.635 gm
Text Solution
|
- Find number of molecules in 11.35 litres SO(2) gas at STP.
Text Solution
|
- 2NH(3) to N(2) + 3H(2) Find moles of H(2) produced from 4 moles of ...
Text Solution
|
- CaCO(3) + 2HCl to CaCl(2) + H(2)O + CO(2) 0.2 mole CaCO(3) is reacte...
Text Solution
|
- NH(2)C"OO"NH(4) overset(Delta)to 2NH(3) + CO(2) If 6 moles of NH(3) i...
Text Solution
|
- KClO(3) to KCl + O(2)(unbalanced), If in above reaction 5 moles of K...
Text Solution
|
- 2NH(3) to N(2) + 3H(2) If at the reaction, 18 mole of H(2) is produ...
Text Solution
|
- A 2000 gm sample of CaCO(3) is 80 % pure. Find weight ( in gram ) of...
Text Solution
|
- An impure sample ( having 60 % purity ) of KClO(3) contains 30 gm of p...
Text Solution
|
- A 200 gm sample of CaCO(3) having 40 % purity is heated. Find moles...
Text Solution
|
- 5 moles of CaCO(3) on heating yeilded 2 moles of CO(2) .Find % yield o...
Text Solution
|
- 245 gm of KClO(3) on heating yielded 64 gm O(2).Find % yield of reacti...
Text Solution
|
- A to 2B + C 3B to 2D Find moles of D produced if initially 3 mole...
Text Solution
|
- {:(2A +3B to 4C + D),(" "darr),(" (excess)"),(3C to 2E):} ...
Text Solution
|
- 2 moles carbon and 1.5 moles of oxygen gas are reacted in a container ...
Text Solution
|