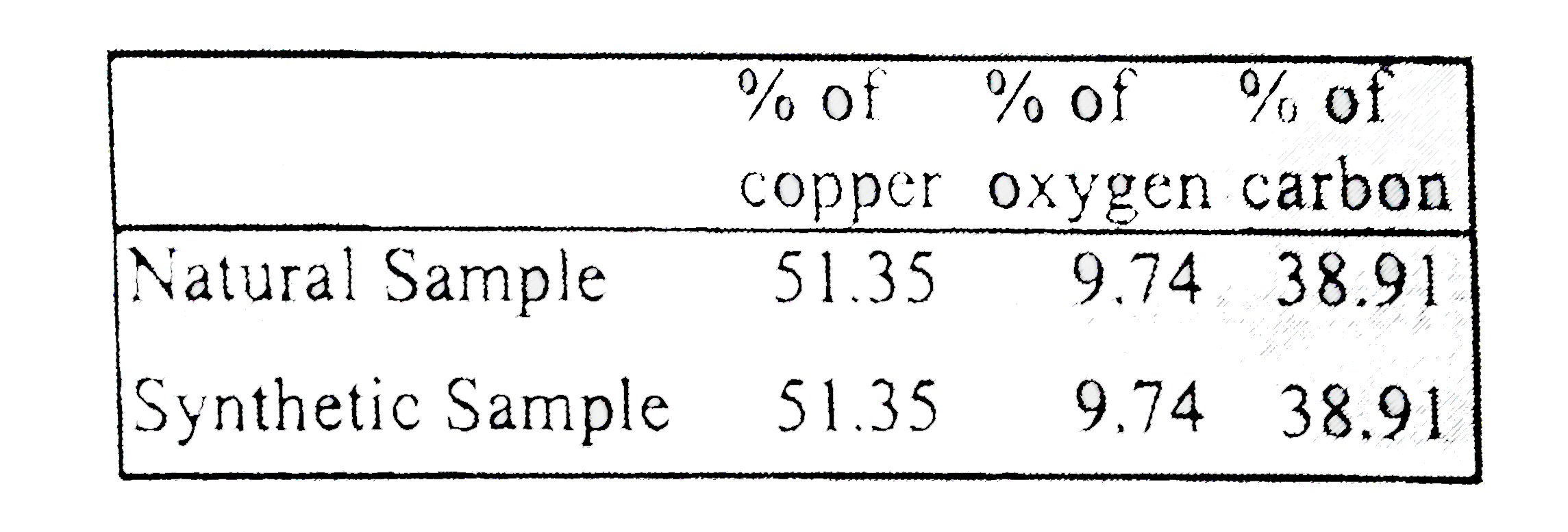Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MOLE CONCEPT
ALLEN|Exercise O-I|45 VideosMOLE CONCEPT
ALLEN|Exercise O-II|11 VideosMOLE CONCEPT
ALLEN|Exercise S-I (Problems Related with Different Types of Atomic Masses & Basic Concept of Mole)|52 VideosMETALLURGY
ALLEN|Exercise EXERCISE-05 [B]|26 Videosp-Block Element
ALLEN|Exercise All Questions|20 Videos
Similar Questions
Explore conceptually related problems
ALLEN-MOLE CONCEPT-S-II
- Two substances P(4) &O(2) are allowed to react completely to form mixt...
Text Solution
|
- By the reaction of carbon and oxygen, a mixture of CO and CO(2) is obt...
Text Solution
|
- Nitrogen (N), phosporus(P), and potassium (K) are the main nutrients i...
Text Solution
|
- A 10 gm. sample of a mixture of calcium chloride and sodium chloride i...
Text Solution
|
- A mixture of ferric oxide (Fe(2)O(3)) and Al is used as a solid rocket...
Text Solution
|
- 5.33 mg of salt [Cr(H(2)O)(5)Cl].Cl(2).H(2)O is treated with excess of...
Text Solution
|
- If mass % of oxygen in monovalent metal carbonate is 48% ,then find th...
Text Solution
|
- To find formula of compound composed of A & B which is given by A(X)B(...
Text Solution
|
- Calculate maximum mass of CaCl(2) produced when 2.4 xx 10^(24)atoms is...
Text Solution
|
- P(4)S(3) + 8O(2) to P(4)O(10) + 3SO(2) Calculate mass of P(4)S(3...
Text Solution
|
- Consider the following equation H(4)P(2)O(7) + 2NaOH to Na(2)H(2)P(...
Text Solution
|
- It states that matter can neither be created nor destroyed. This law...
Text Solution
|
- It states that matter can neither be created nor destroyed. This law...
Text Solution
|
- This law was given by, a French chemist , Joseph Proust. He stated tha...
Text Solution
|
- This law was given by, a French chemist , Joseph Proust. He stated tha...
Text Solution
|
- This law was proposed by Dalton in 1803 . According to this law , if t...
Text Solution
|
- This law was proposed by Dalton in 1803 . According to this law , if t...
Text Solution
|
- This law was proposed by Dalton in 1803 . According to this law , if t...
Text Solution
|