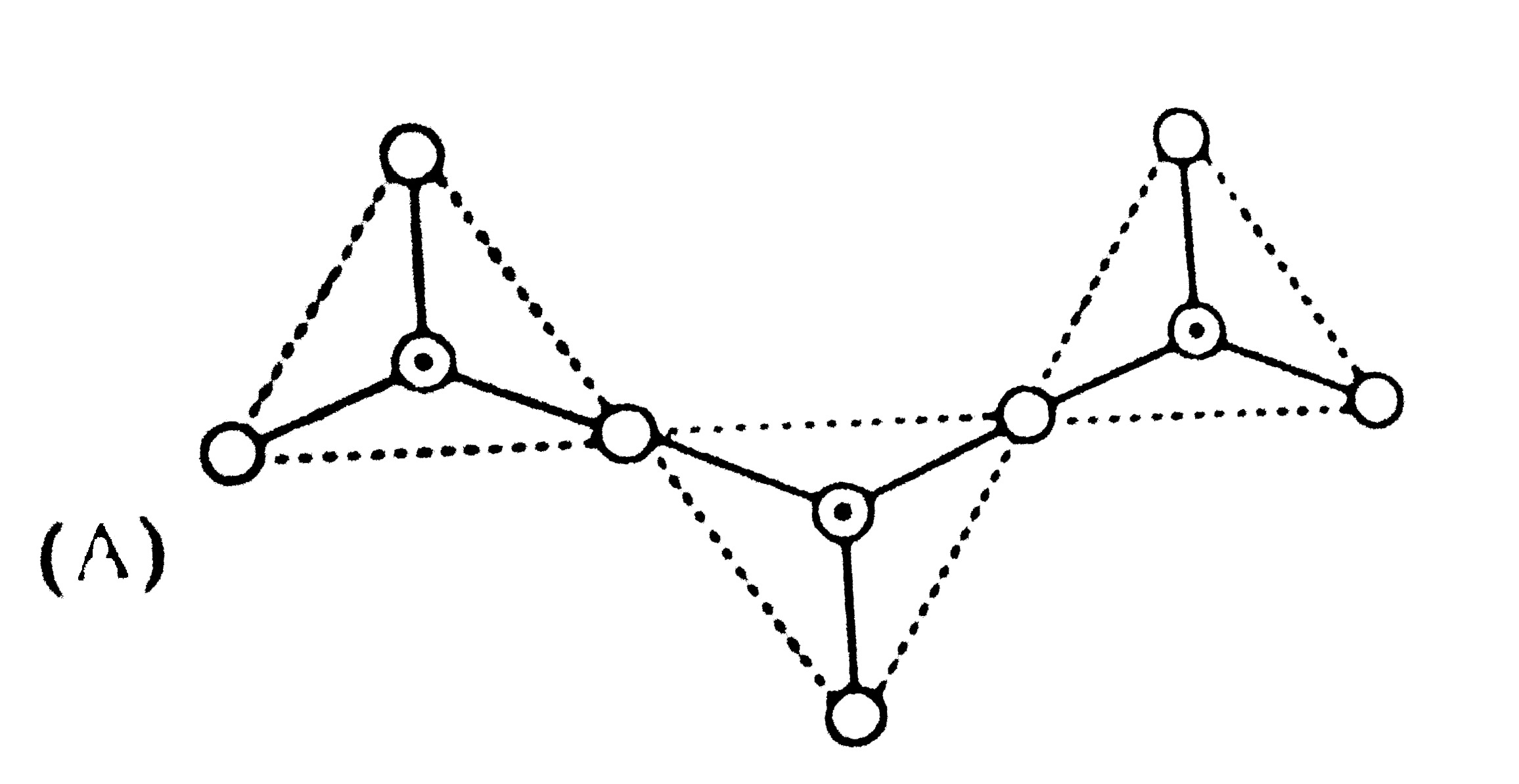
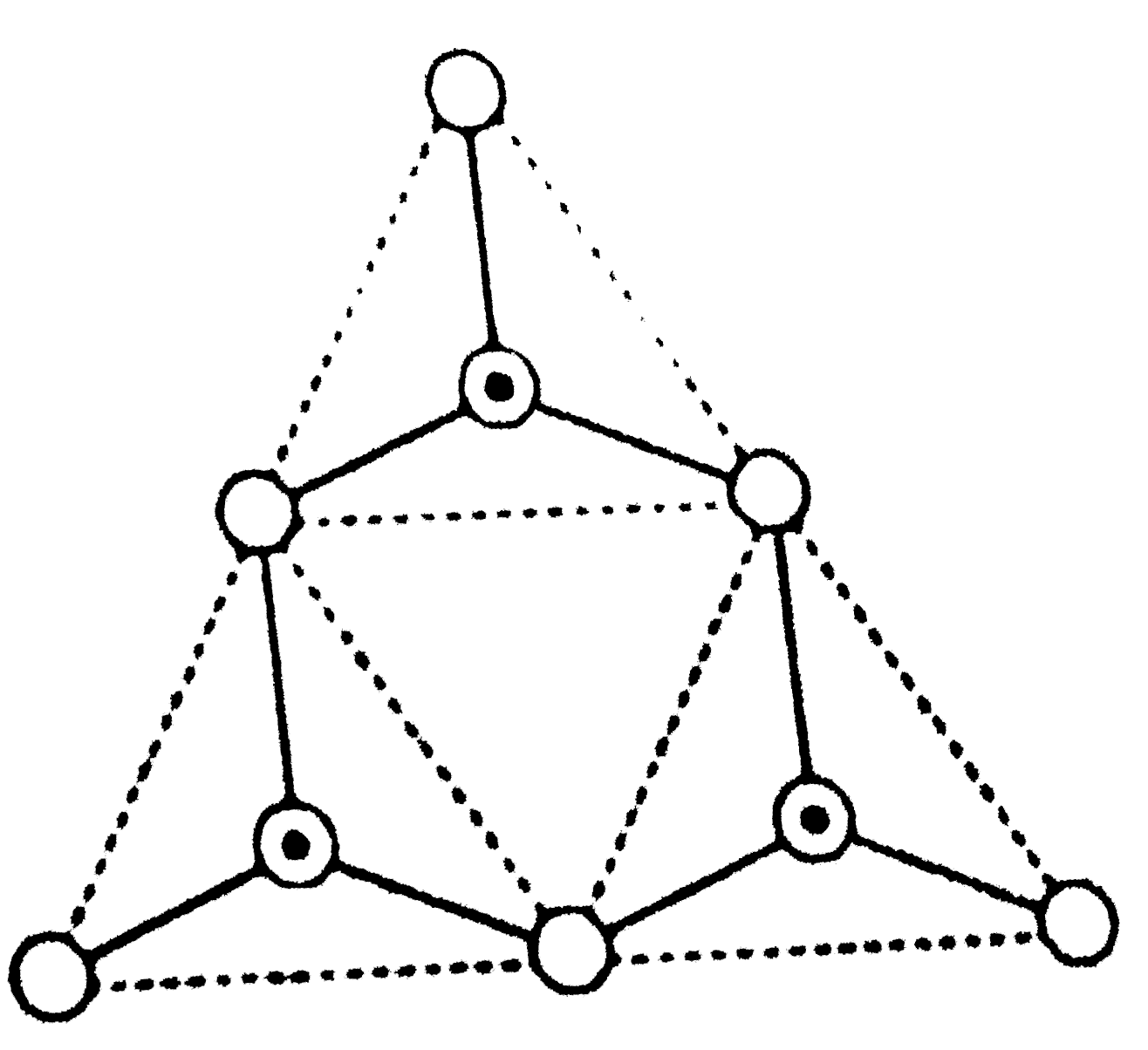
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The name 'silica' covers an entire group of minerals which have the ge...
Text Solution
|
- The name 'silica' covers an entire froup of minerals which have the ge...
Text Solution
|
- The name 'silica' covers an entire froup of minerals which have the ge...
Text Solution
|
- Read the following short write-up and answer the questions at the end ...
Text Solution
|
- Read the following short write-up and answer the questions at the end ...
Text Solution
|
- Read the following short write-up and answer the questions at the end ...
Text Solution
|
- Read the following short write-up and answr the questions at the end o...
Text Solution
|
- Read the following short write-up and answr the questions at the end o...
Text Solution
|
- Read the following short write-up and answr the questions at the end o...
Text Solution
|