A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ALKENE, ALKANE & ALKYNE -EXERCISE-5
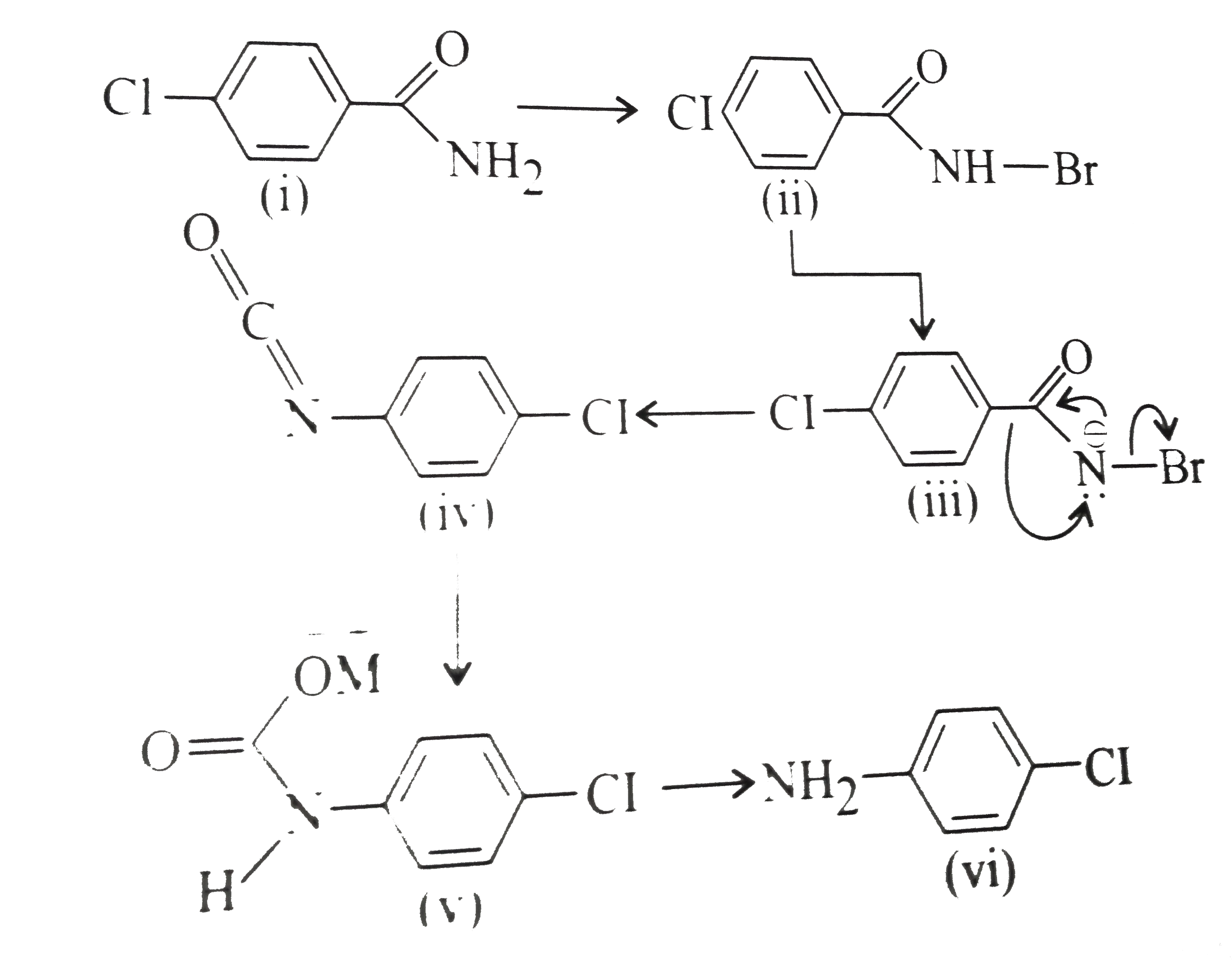
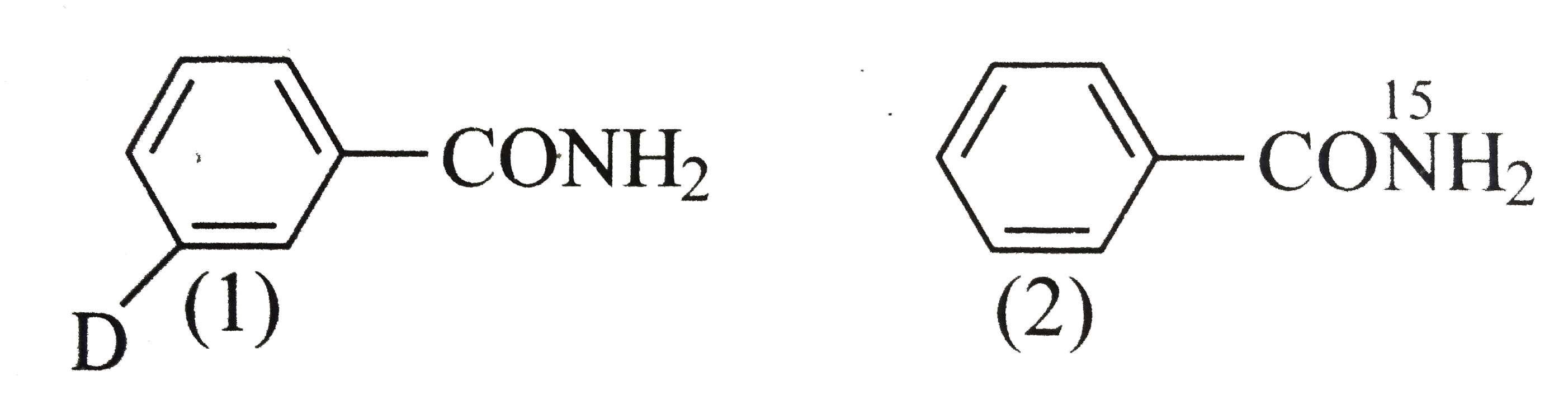
- RCONH2 is converted into RNH2 by means of Hofmann bromamide degradatio...
Text Solution
|
- RCONH2 is converted into RNH2 by means of Hofmann bromamide degradatio...
Text Solution
|
- RCONH2 is converted into RNH2 by means of Hofmann bromamide degradatio...
Text Solution
|
- Gases released in reaction (I) and (II) are:
Text Solution
|
- Order of boiling point for the following compounds is-
Text Solution
|
- Reimer-Tiemann reaction introduces an aldehyde group on to the aromati...
Text Solution
|
- Reimer-Tiemann reaction introduces an aldehyde group on to the aromati...
Text Solution
|
- Reimer-Tiemann reaction introduces an aldehyde group on to the aromati...
Text Solution
|
- Reimer-Tiemann reaction introduces an aldehyde group on to the aromati...
Text Solution
|
- Statement 1: In bromobenzene upon reaction with Br(2)//Fe gives ,4 dib...
Text Solution
|
- Statement I: Aniline on reaction with NaNO2 HCl at 0^@C followed by c...
Text Solution
|
- In the reaction is/are:
Text Solution
|
- Compounds (P), (Q) and (S) were separetly subjected to nitration ...
Text Solution
|
- The correct order of strength of acidity of the following compounds is
Text Solution
|
- In the reaction, the structure of the product T is:
Text Solution
|
- Among the following compounds the most acidic is
Text Solution
|
- Compound (A), C(8)H(9)Br, gives a white precipitate when warmed with a...
Text Solution
|
- In the reaction shown below, the major product (s) formed is/are
Text Solution
|
- The reactivity of compound Z with different halogens under appropriat...
Text Solution
|
- Consider the reaction sequence below:
Text Solution
|
_E01_301_O01.png)
_E01_301_O02.png)
_E01_301_O03.png)
_E01_301_O04.png)