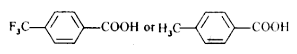Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ALKENE, ALKANE & ALKYNE -EXERCISE-4[A]
- Give reasons for the following : 'Carbon-oxygen bond lengths in form...
Text Solution
|
- Assertion:- pKa(1) of fumaric acid is greater than maleic acid. Reas...
Text Solution
|
- Which is stronger conjugate base in each pair ? (A) overset(-)OH or ...
Text Solution
|
- Which acid of each pair shown here would you expect to be stronger? ...
Text Solution
|
- What are A and B in the following ?
Text Solution
|
- In the following reaction, trace the position of isotopic O^(18) CH(...
Text Solution
|
- Write the reagents to carry out following conversions: CH(3)overset(...
Text Solution
|
Text Solution
|
- What are X and Y ?
Text Solution
|
- Write down the structure of A? What is the use of A ?
Text Solution
|
- Arrange the following in decreasing basic order with proper reasoning.
Text Solution
|
- Sulphanilic acid is a/an:
Text Solution
|
- What is the order of basicity of the following compounds ? (i) CH(3)...
Text Solution
|
- What is the order of basicity of the following compounds ? (i) CH(3)...
Text Solution
|
- Unlike other aromatic amines, why is the following amine stongly basic...
Text Solution
|
- In this compound which site acts as an acid and which as a base ?
Text Solution
|
- Alkyl cyanides (CH(3)CN) when treated with hydrogen in presence of P...
Text Solution
|
- Write the compound (A) and (B) formed in this
Text Solution
|
- Identify the stronger base in each of the following pairs : (i) CH(3...
Text Solution
|
- In the reaction, C(6)H(5)NH(2)underset(0-5^(@)C)overset(NaNO(2)+HCl)...
Text Solution
|