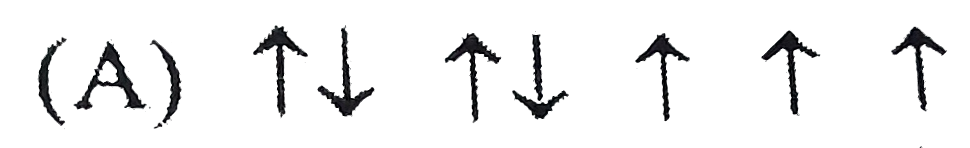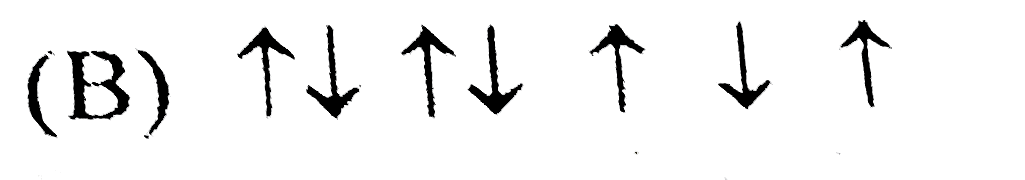A
B
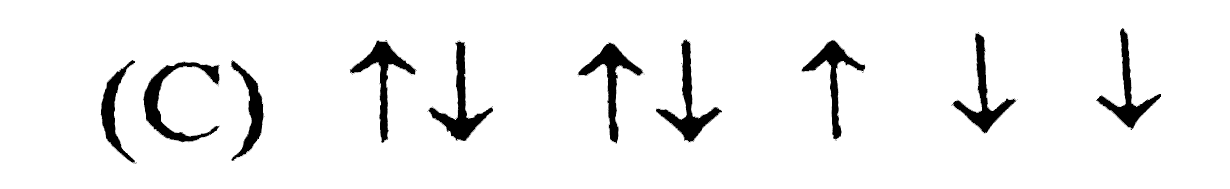
C
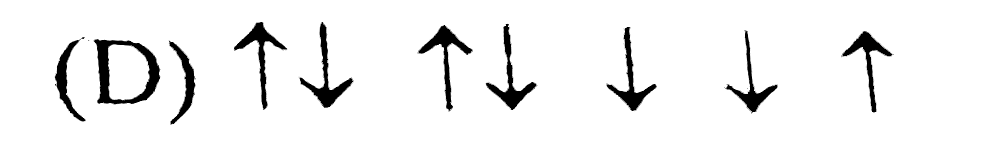
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-QUANTUM NUMBER & PERIODIC TABLE-J-ADVANCED EXERCISE
- The basic nature of hydroxides of group 13 (III-A) decreases progressi...
Text Solution
|
- Moving from right to left in a periodic table, the atomic size is :
Text Solution
|
- The increasing order of electronegativity in the following elements :
Text Solution
|
- One element has atomic weight 39. Its electronic configuration is 1s^(...
Text Solution
|
- The number of paired electrons in oxygen atom is :
Text Solution
|
- The decreasing size of K^(+),Ca^(2+),Cl^(-) & S^(2-) follows the order...
Text Solution
|
- The incorrect statement Among the following is A)The first ionisation...
Text Solution
|
- Li^(+),Mg^(2+),K^(+),Al^(3+) (Arrange in increasing order of radii)
Text Solution
|
- Which one of the following statement (s) is (are) correct?
Text Solution
|
- The ground state electronic configuration of nitrogen atom can be repr...
Text Solution
|
- The electronic configuration of an element is 1s^(2)2s^(2)2p^(6)3s^(2)...
Text Solution
|
- Assertion: F atom has less negative electron gain enthaply than Cl ato...
Text Solution
|
- The correct order of radii is
Text Solution
|
- The IE of Be is greater than that of B.
Text Solution
|
- The set representing correct order of IP(1) is
Text Solution
|
- The least stable ion among the following is
Text Solution
|
- The maximum number of electrons that can have principal quantum number...
Text Solution
|
- In an atom, the total number of electrons 'having quantum numbers' n =...
Text Solution
|
- The correct order of atomic radii in group 13 elements is
Text Solution
|
- The option (s) with only amphoteric oxides is (are)
Text Solution
|