Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BODY FLUIDS AND CIRCULATION
ALLEN|Exercise Labelling based question|5 VideosBODY FLUIDS AND CIRCULATION
ALLEN|Exercise Matrix Type|10 VideosBODY FLUIDS AND CIRCULATION
ALLEN|Exercise True False|1 VideosBIOTECHNOLOGY AND MICROBES IN HUMAN WELFARE
ALLEN|Exercise ExerciseII|18 VideosBREATHING AND EXCHANGE OF GASES
ALLEN|Exercise S|33 Videos
Similar Questions
Explore conceptually related problems
ALLEN-BODY FLUIDS AND CIRCULATION-Figure Based Question
- a. In the above given flowchart blood filled in the heart is:- b. Th...
Text Solution
|
- a. The P wave represent the electrical excitation of the b. The QRS ...
Text Solution
|
- "gt
Text Solution
|
- Figure A and B represents the section of which types of blood vessels ...
Text Solution
|
- A and B are:-
Text Solution
|
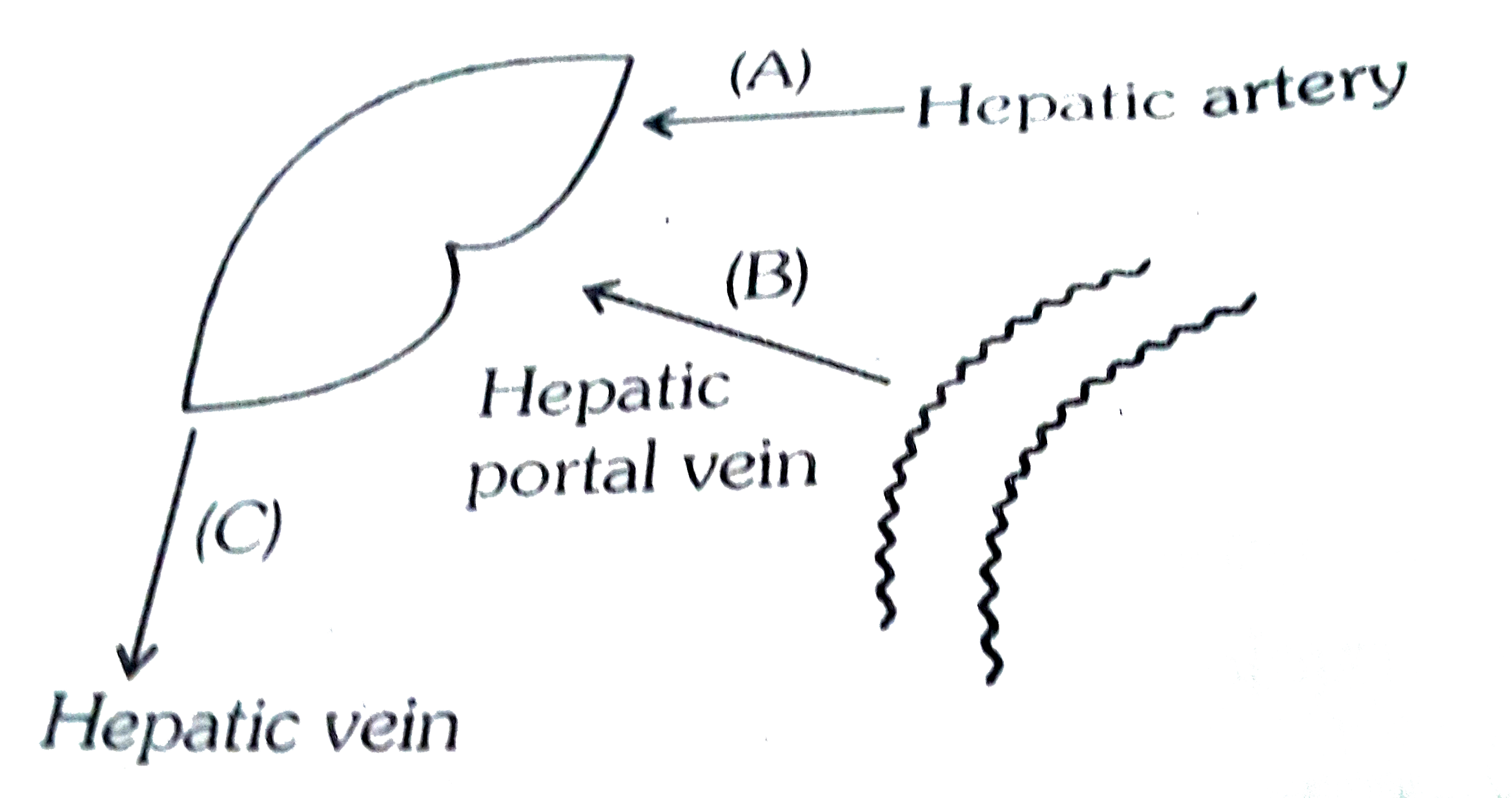
- Identify A,B,C,D in the above given figure:-
Text Solution
|
- which is the correct representation of cardiac cycle.
Text Solution
|