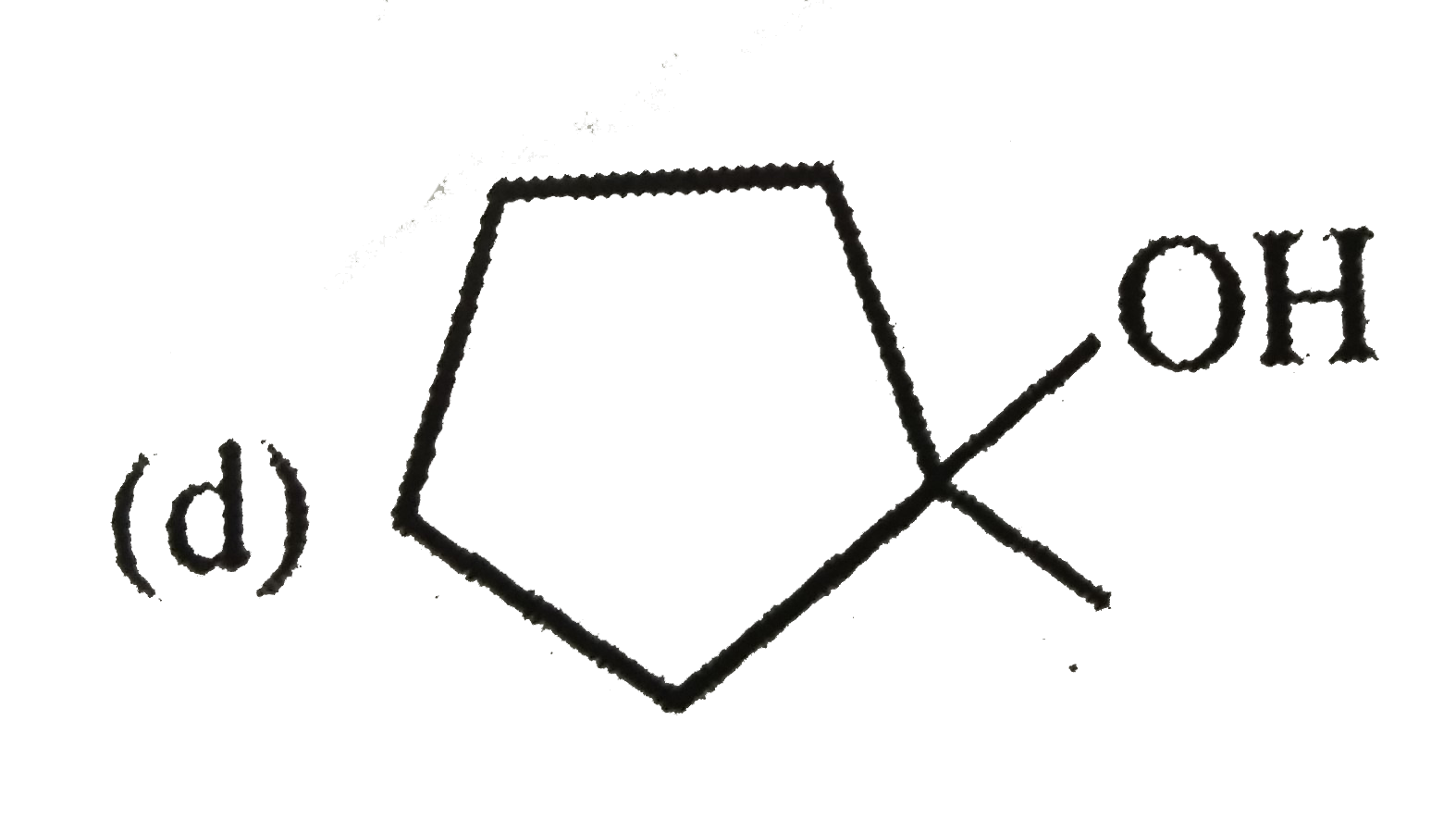
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-BASIC INTRODUCTION AND NOMENCLATURE -MCQ
- Which of the following statements is/are true about a homologous serie...
Text Solution
|
- Number of hetero atoms present in the given compound is :
Text Solution
|
- In following compound CH(3)-CH(2)-underset(CH(3))overset(CH(3))und...
Text Solution
|
- Which of the following compound have maximum number of olefinic bond...
Text Solution
|
- Tertiary butyl amine is a
Text Solution
|
- Vinyl carbinol is
Text Solution
|
- Number of 1^(@), 2^(@), 3^(@) and 4^(@)C present in compound are res...
Text Solution
|
- Classify the following alcohols as primary, secondary, or tertiary :
Text Solution
|
- Number of functional groups present in the following compound is :
Text Solution
|
- Give common name of following compound :
Text Solution
|
- Write down the IUPAC names of the following compounds :- CH(3)-unde...
Text Solution
|
- The structure of isobutyl group in an organic compound is
Text Solution
|