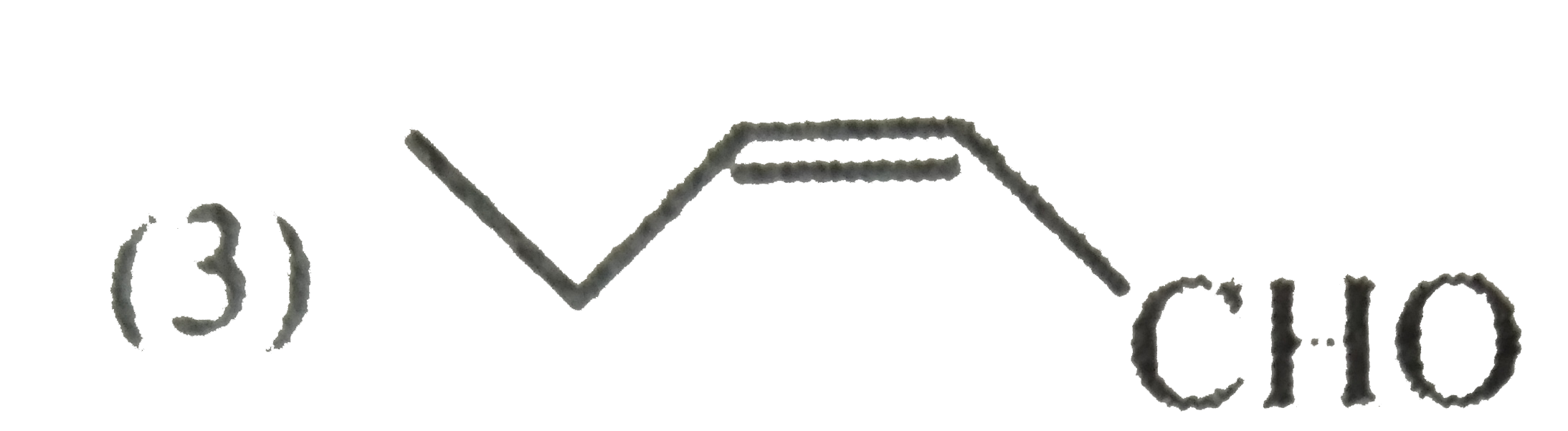
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ISOMERISM -MCQ
- CH(3)CH(2)CH(CHO)CH(2)CH(3) and CH(3)CH(CHO) CH(2)CH(2)CH(3) are :-
Text Solution
|
- Find out relation between the following compounds: CH(3)CH(2)CH(2)un...
Text Solution
|
- How many amines are possible with the molecular formula C(4)H(11)N (...
Text Solution
|
- How many benzenoid isomers are possible for molecular formula C(7)H(8)...
Text Solution
|
- The relation between given compound is :-
Text Solution
|
- The structure below are :-
Text Solution
|
- How many alkanes (only structural isomers) are possible with the molec...
Text Solution
|
- Which of the following is correct
Text Solution
|
- Whether the given compounds can show geometrical isomerism : Pent...
Text Solution
|
- Assign the configuration E/Z to the following compounds.
Text Solution
|
- Which of the following compounds have non zero dipole moment.
Text Solution
|
- Which one the following is an Z isomer ?
Text Solution
|
- Compare dipole moment of the following CH(3)-CH(2)-C=CH ...
Text Solution
|
- Which of the following is a pair of metamers ?
Text Solution
|
- Identify relation that is incorrect
Text Solution
|
- How many structural isomeric alkene possible for molecule formula C5H1...
Text Solution
|
- Which of the following is pair of functional isomers ?
Text Solution
|
- . Which of the following does not show geometrical isomerism?
Text Solution
|
- For compound, which statement is correct
Text Solution
|
- Identify structure of trans-2-hexenal.
Text Solution
|