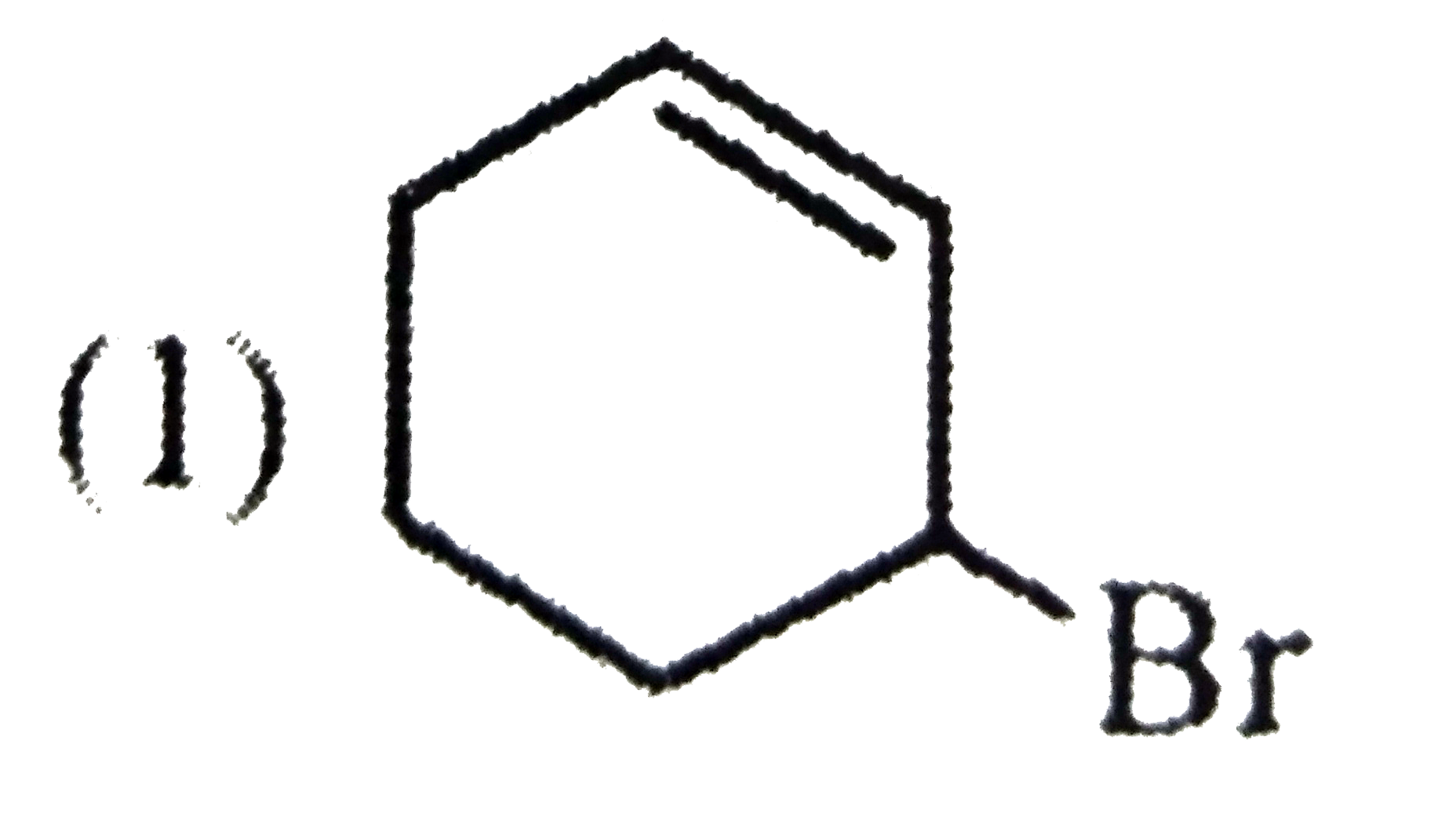
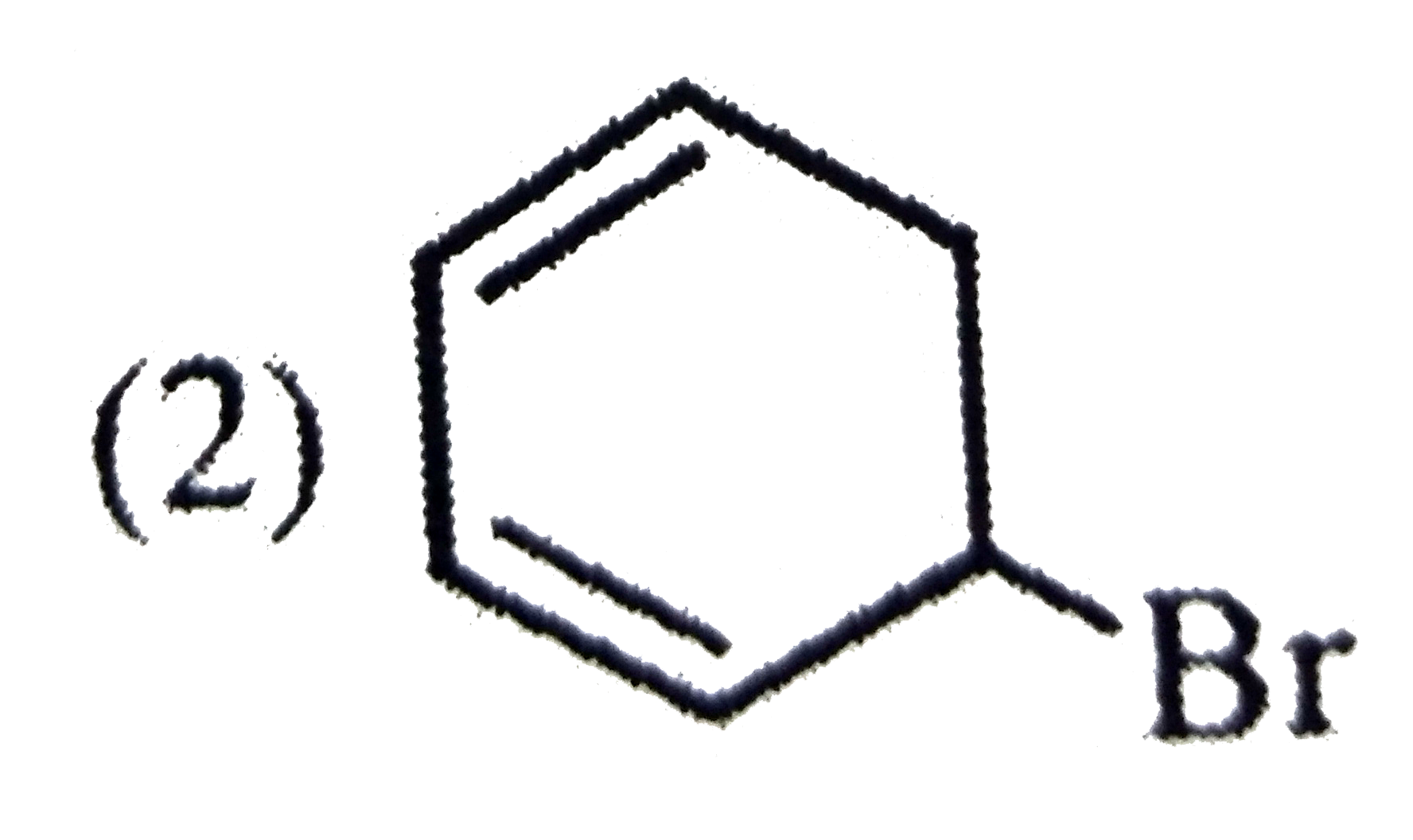
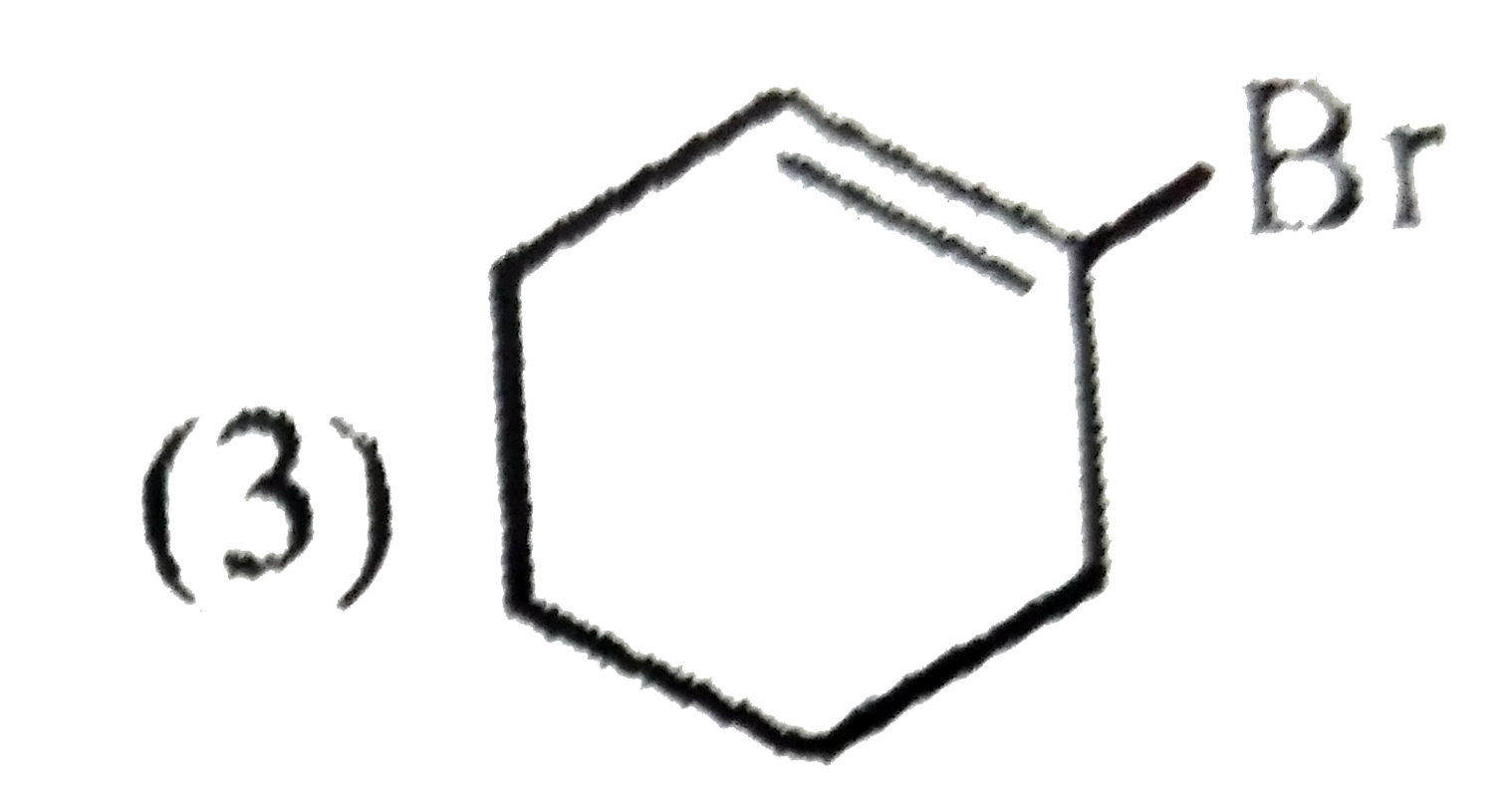
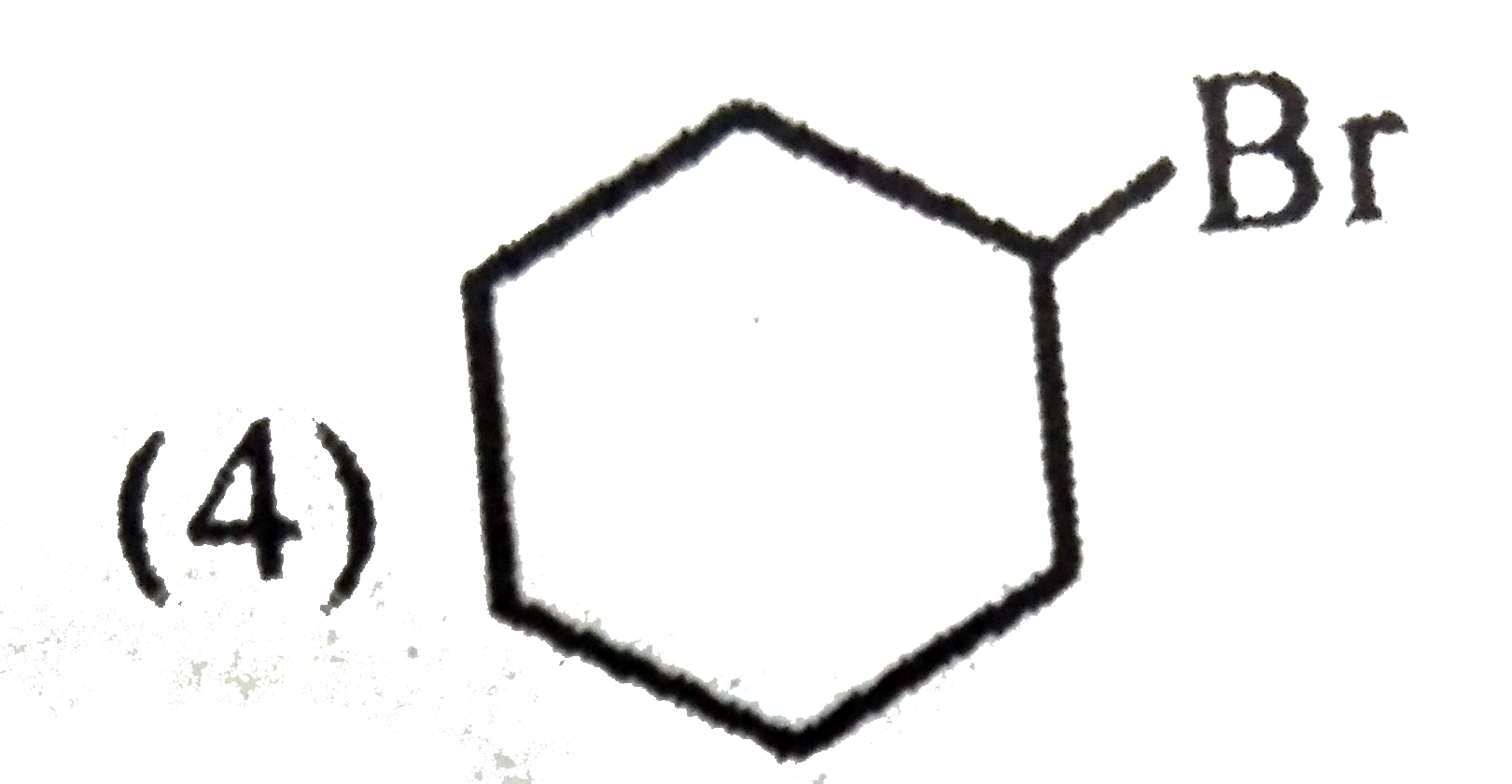
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-REACTION MECHANISM (PART-II)-MCQ
- Arrange the following compound in reactivity towards EAS (Electrophili...
Text Solution
|
- Arrange .the following in increasing order of stability of carbocation...
Text Solution
|
- Draw the resonance structures of the following compounds. (a) CH(2) ...
Text Solution
|
- Name of the following as substituted derivatives of ethylene. (a) C...
Text Solution
|
- Which of the following is maximum reactive for dehydrohalogenation :-
Text Solution
|
- Complete the following reaction
Text Solution
|
- The decreasing order of bond dissociation energy of C-H bond in given ...
Text Solution
|
- Match column-I and column-II and select the answer :
Text Solution
|
- Arrange the following in decreasing order of rate of nitration :-
Text Solution
|
- Identify the product of following reaction ?
Text Solution
|
- Match the column and select the answer :-
Text Solution
|
- A and B respectively are :-
Text Solution
|
- What are the major product of following reaction ?
Text Solution
|
- Which of the following alcohols is dehydrated most easily with conc. H...
Text Solution
|