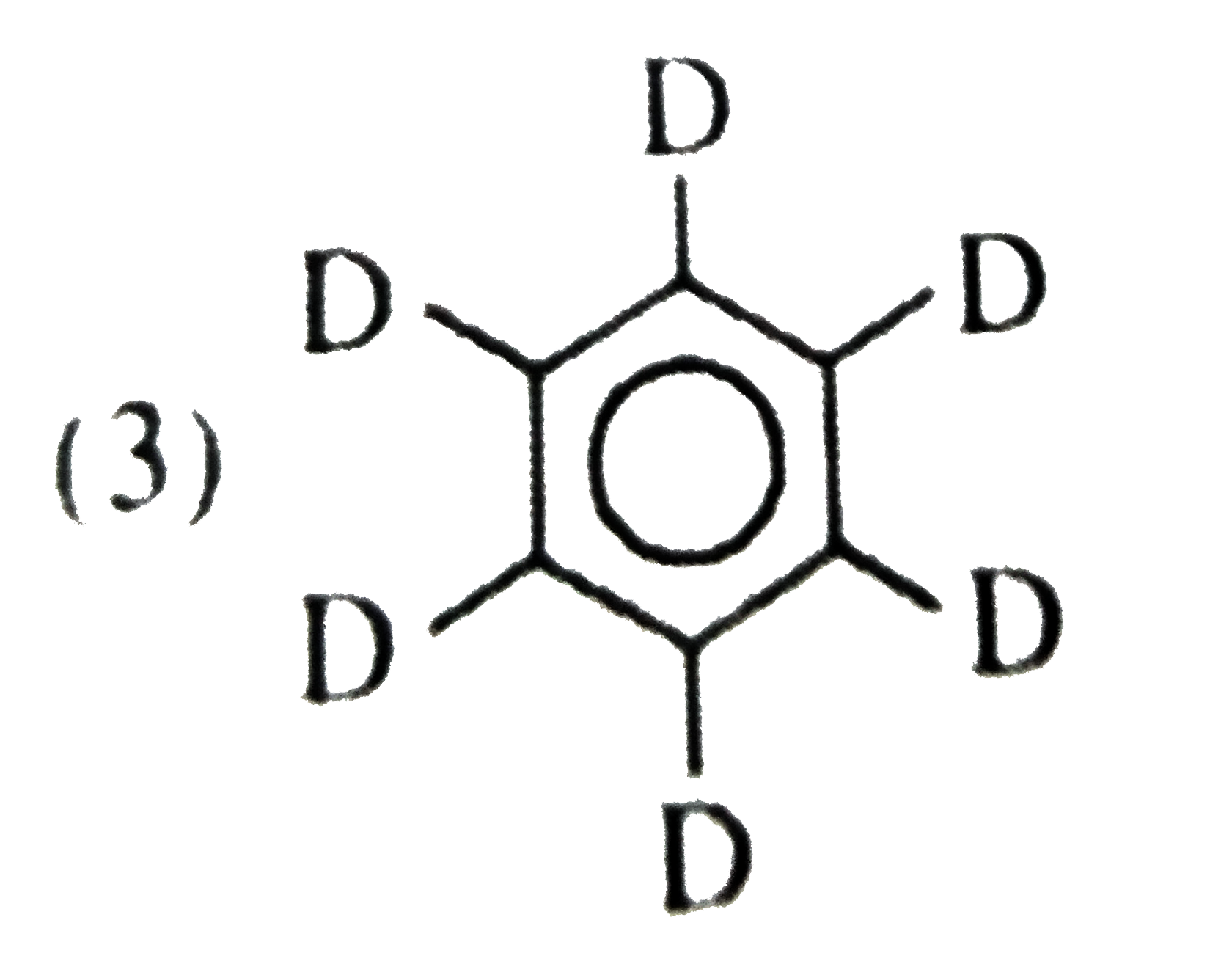
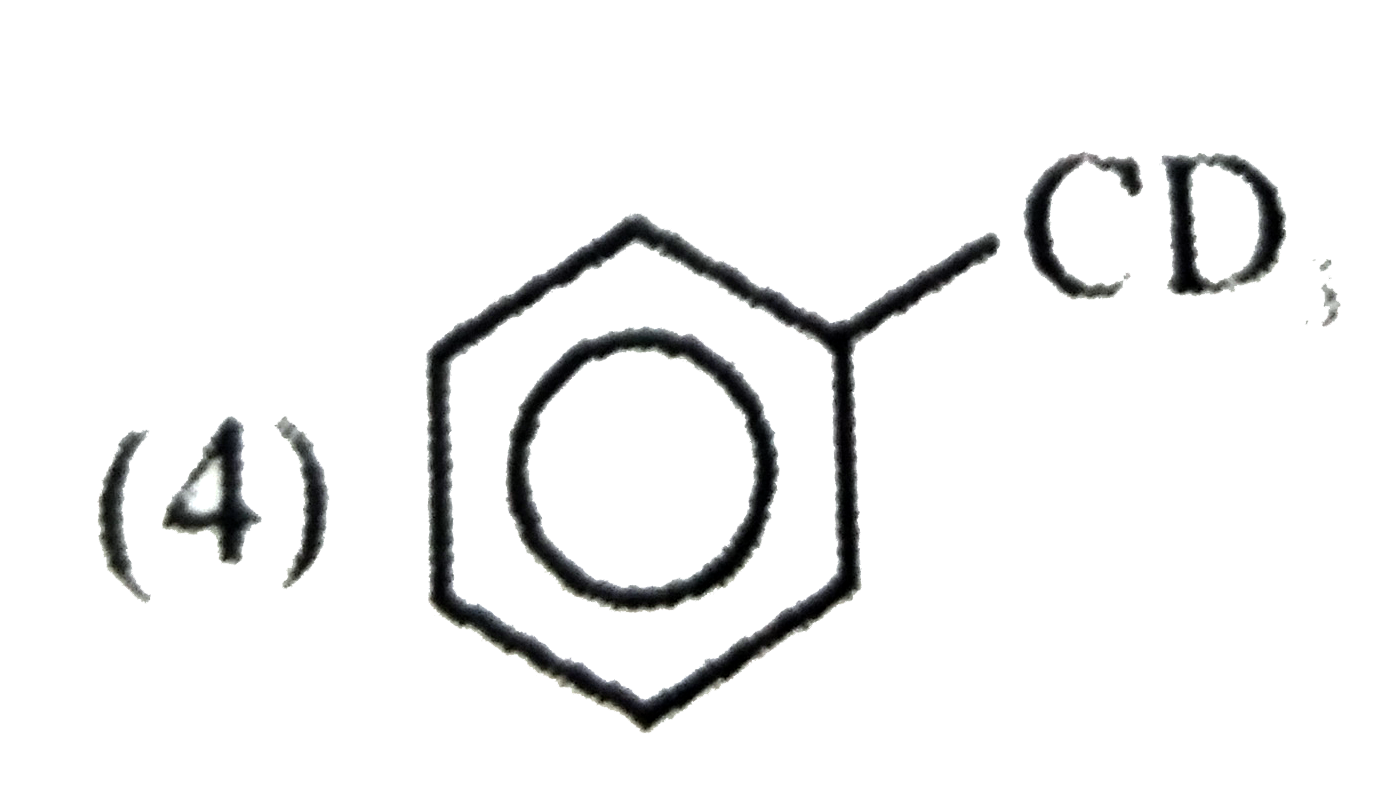
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-HYDROCARBON-MCQ
Text Solution
|
- What would be the product formed when 1-bromo-3-chlorocylobutane rea...
Text Solution
|
- CH(2)OHCH(2)CH(2)COCH(3)overset("Reagent")rarr CH(2)OHCH(2)CH(2)C...
Text Solution
|
- Which of the following alkane is synthesised from single alkyl halide ...
Text Solution
|
- Name the functional groups present in the following compounds. (a) ...
Text Solution
|
- The compound that decolourises alk. KMnO4 is
Text Solution
|
- Identify the hydrocarbon which on ozonolysis gives :- (a) Only ac...
Text Solution
|
- The reaction that give good yield of n-Hexane is :-
Text Solution
|
- underset("Calcium carbide")(CaC(2))overset(H(2)O)(to)(A)overset("Red h...
Text Solution
|
- IUPAC name of the following compound is CH(3)-CH=CH-CH(2)-C-=CH
Text Solution
|
- Birch reduction.
Text Solution
|
- But-1-yne and But-2-yne can be distinguished by :
Text Solution
|
- Give product of the following reactions :-
Text Solution
|
- A overset("Kolbe's electrolysis")rarr propyne will be
Text Solution
|
- If X and Y are moles used then sum of |X+Y=| is :-
Text Solution
|