A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-Chemical Equilibrium-All Questions
- K(p) value for C(2)H(4(g))+H(2(g))rArrC(2)H(6(g))is 5xx10^(18)" a...
Text Solution
|
- E(cell)^(@) for the reaction MnO(4)^(-)+5Fe^(+2)+8H^(+)rarrMn^(+2)+5Fe...
Text Solution
|
- The conversion of into oxygen is exothermic. Under what conditions is ...
Text Solution
|
- Consider the following gas - phase reaction : 2A(g)+B(g)rArrC(g)...
Text Solution
|
- In the dissociation of I(2)(g) at 1000 K in a container of 1 litre :-...
Text Solution
|
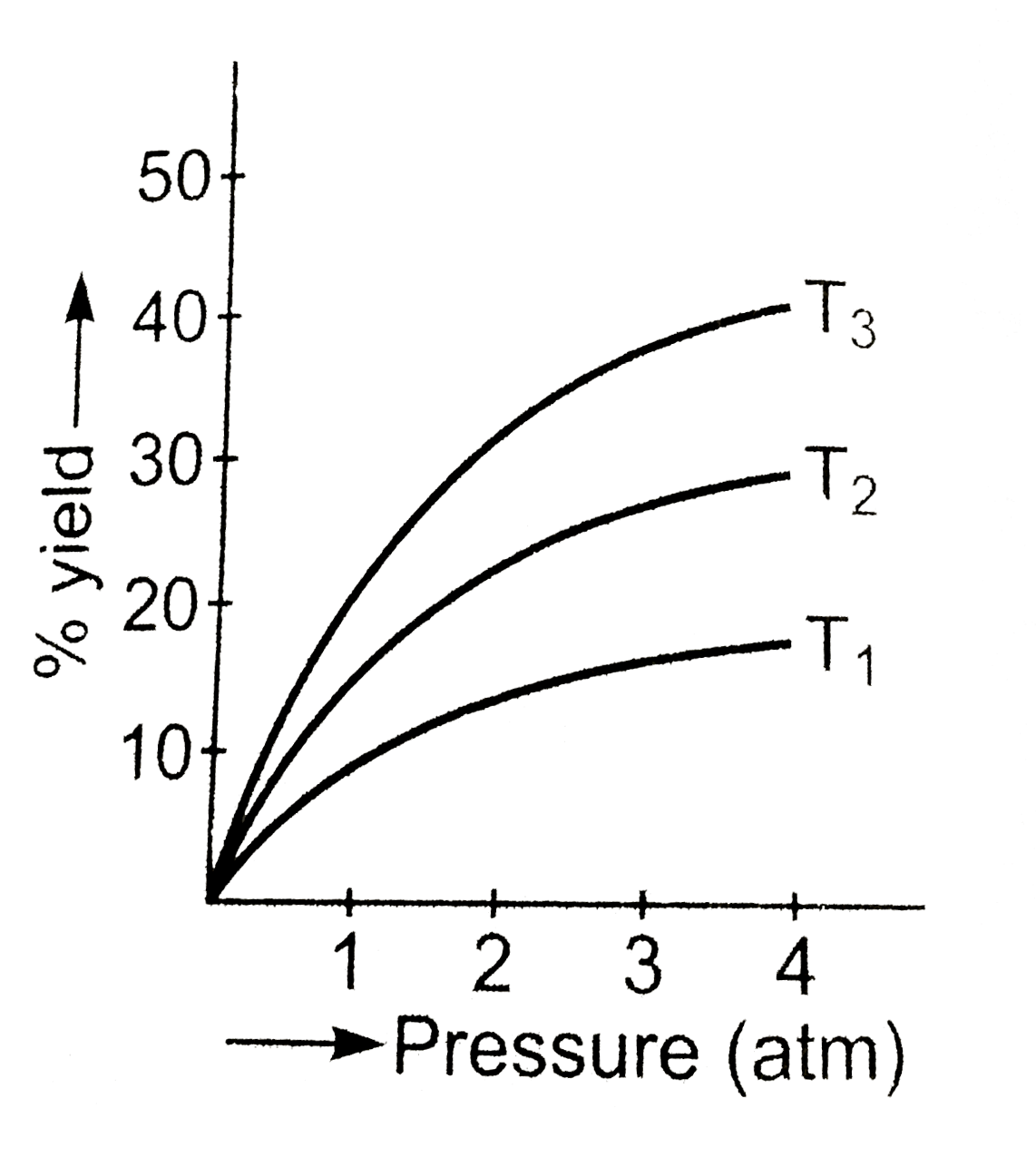
- What is the effect of temperature and pressure on the yields of produc...
Text Solution
|
- In a reversible reaction K(c)gtK(p)andDeltaH=+40kCal. The product w...
Text Solution
|
- For the dissociation reaction N(2)O(4) (g)hArr 2NO(2)(g), the degree o...
Text Solution
|
- The prepation of SO(3)(g) by reaction SO(2)(g)+(1)/(2)O(2)(g)hArrSO(3)...
Text Solution
|
- The equilibrium constant for : PCl(5)rArrPCl(3)+Cl(2) is 0.5 a...
Text Solution
|
- At 473 K,K(c) for the reaction PCl(5(g))rArrPCl(3(g))Cl(2(g)) ...
Text Solution
|
- 18.4 g of N(2)O(4) is taken in a 1 L closed vessel and heated till the...
Text Solution
|
- 5 moles of SO(2) and 5 moles of O(2) are allowed to react to form SO(3...
Text Solution
|
- For the reaction N(2(g))+O(2(g))hArr2NO((g)), the value of K(c) at 800...
Text Solution
|
- For the reaction a+bhArrc+d, initially concentrations of a and b are e...
Text Solution
|
- NH(4)CONH(2)(s)hArr2NH(3)(g)+CO(2)(g). If equlibrium pressure is 3 atm...
Text Solution
|
- 0.6 mole of PCl(5), 0.3 mole of PCl(3) and 0.5 mole of Cl(2) are taken...
Text Solution
|
- N(2)O(4(g))rArr2NO(2),K(c)5.7xx10^(-9) at 298 K At equilibrium :-
Text Solution
|
- The following reaction is at equilibrium , underset("Yellow")(Fe(...
Text Solution
|
- Formation of ClF(3) from Cl(2)and F(2) is an exothermic process . The ...
Text Solution
|