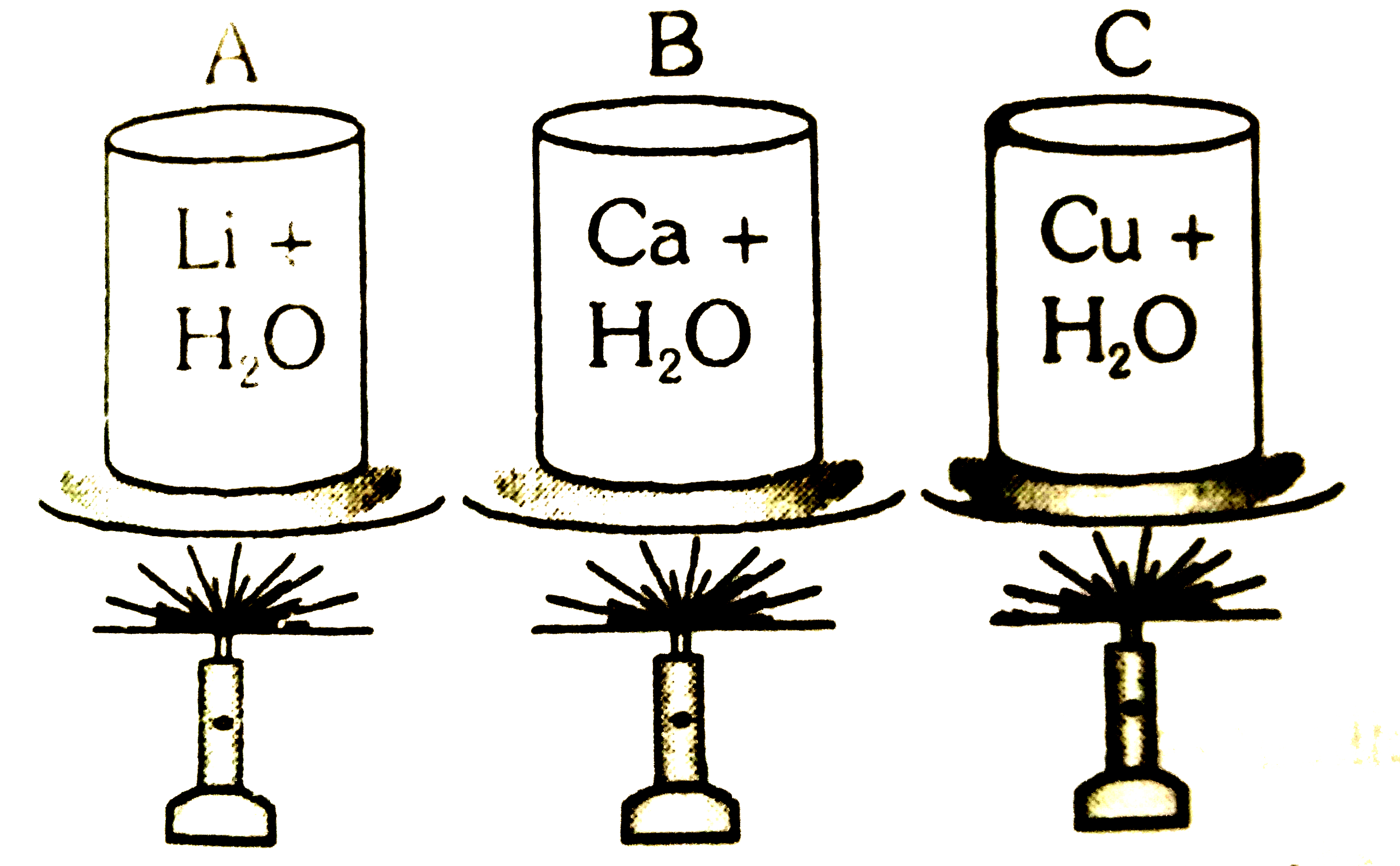A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-HYDROGEN-EXERCISE-1
- Weakest reducing agent:
Text Solution
|
- Nuclear isomerism is exhibited by-
Text Solution
|
- Which of the following statement is not true for .(1)H^(1),.(1)H^(2),....
Text Solution
|
- Hydrogen has three isotopes, the number of possible diatomic molecules...
Text Solution
|
- Dihydrogen has :
Text Solution
|
- Hydrogen is -
Text Solution
|
- In which property listed below hydrogen does not resemble alkali metal...
Text Solution
|
- In which of the following reaction dihydrogen acts as an oxidising age...
Text Solution
|
- Which of the following is an nuclear isomer of hydrogen ?
Text Solution
|
- The correct order of reactivity among I (atomic hydrogen), II (Dihyd...
Text Solution
|
- Which combination cannot be used for the preparation of hydrogen gas i...
Text Solution
|
- By which reaction. Best yield of H(2) gas forms : Temperature of ...
Text Solution
|
- By which reaction best yield of H(2) gas forms: temperature of al...
Text Solution
|
- Hydrogen has the tendency to lose one e^(-) and formation of H^(+), In...
Text Solution
|
- At sun atmosphere which of the following forms is stable :
Text Solution
|
- Ratio of Ortho & Para hydrogen in ordinary hydrogen is :
Text Solution
|
- H(2) gas can not be prepared by :
Text Solution
|
- Deuterium is an isotope of hydrogen.
Text Solution
|
- H(2) gas is liberated at cathode and anode both by electrolysis of the...
Text Solution
|
- Under what conditions of temperature and pressure the formation of at...
Text Solution
|