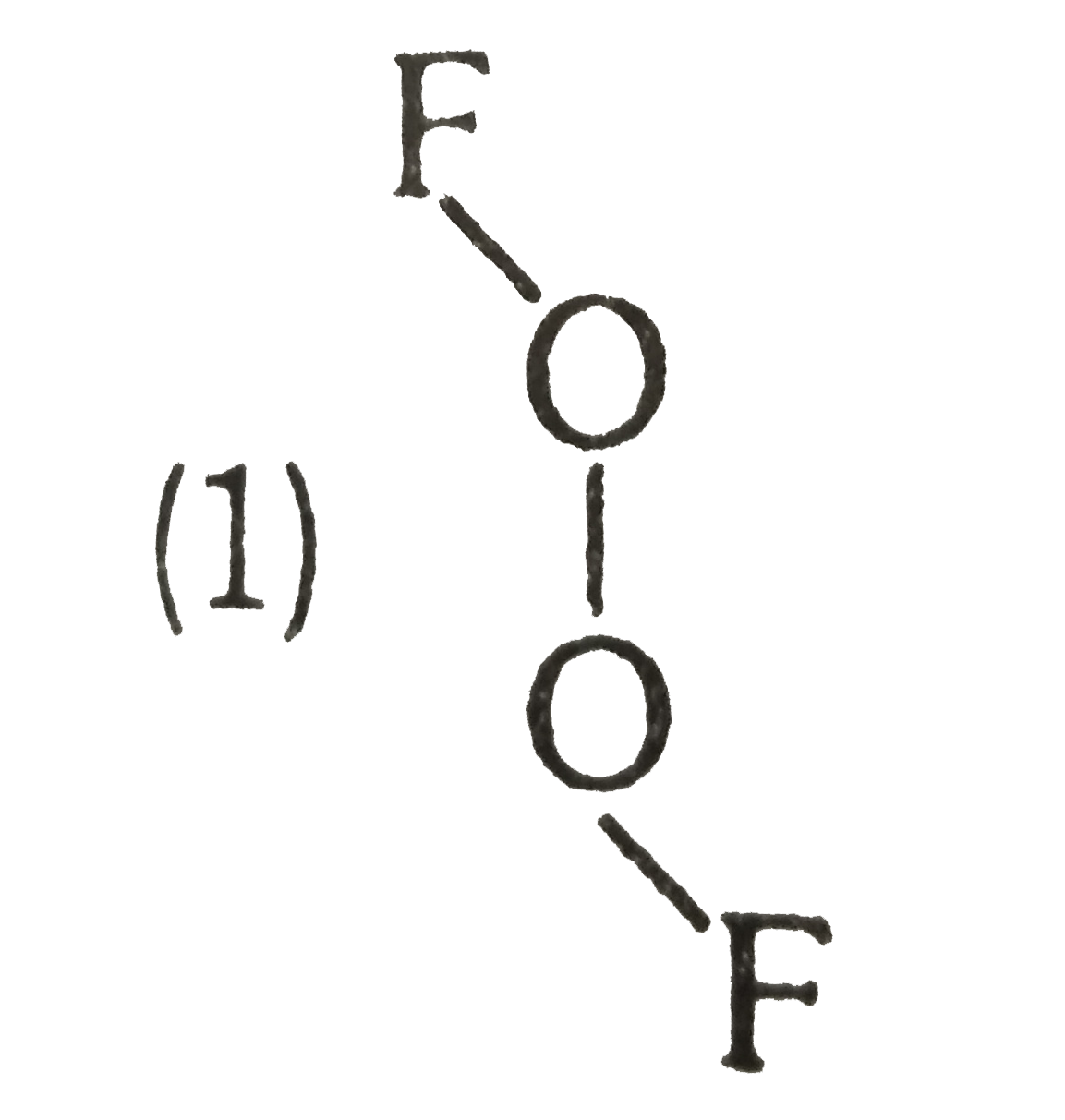A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-HYDROGEN-EXERCISE-3
- H(2)O(2) is used but not as:
Text Solution
|
- Which is accepted structure of H(2)O(2) is gas phase :
Text Solution
|
- HCl is added to the following oxides, which one would give H(2)O(2)?
Text Solution
|
- A mixture of hydazine (N(2)H(4)) and 58-60% solution of H(2)O(2) i...
Text Solution
|
- Which of the following is a true structure of H(2)O(2)?
Text Solution
|
- In the reaction 2H(2)O(2)rarr2H(2)O+O(2) oxidation state of oxygen cha...
Text Solution
|
- The dipole moment of H(2)O(2) is 2.1 D. this indicates that the struct...
Text Solution
|
- H(2)O(2) and heavy water was discovered by respectively :
Text Solution
|
- The dihedral angle of H(2)O(2) in solid phase is
Text Solution
|
- Hydrogen peroxides cannot be concentrated easily because
Text Solution
|
- An aqueous solution of H(2)O(2)
Text Solution
|
- Which of the following statements about H(2)O(2) is not true ?
Text Solution
|
- Bleaching action of H(2)O(2) is due to its :
Text Solution
|
- In the Merck's process the reagents involved for the preparation of hy...
Text Solution
|
- H(2)O(2) generally conllected in:
Text Solution
|
- Acidic order of following compounds is : (I) H(2)O(2)," (I...
Text Solution
|
- Which of following true structure of F(2)O(2):
Text Solution
|
- Correct ORDER of acidity & dielectric constant is
Text Solution
|
- Correct order of BP is :-
Text Solution
|
- Decomposition of H(2)O(2) is retarded by : 2H(2)O(2)(l) rarr 2H(2)O(...
Text Solution
|