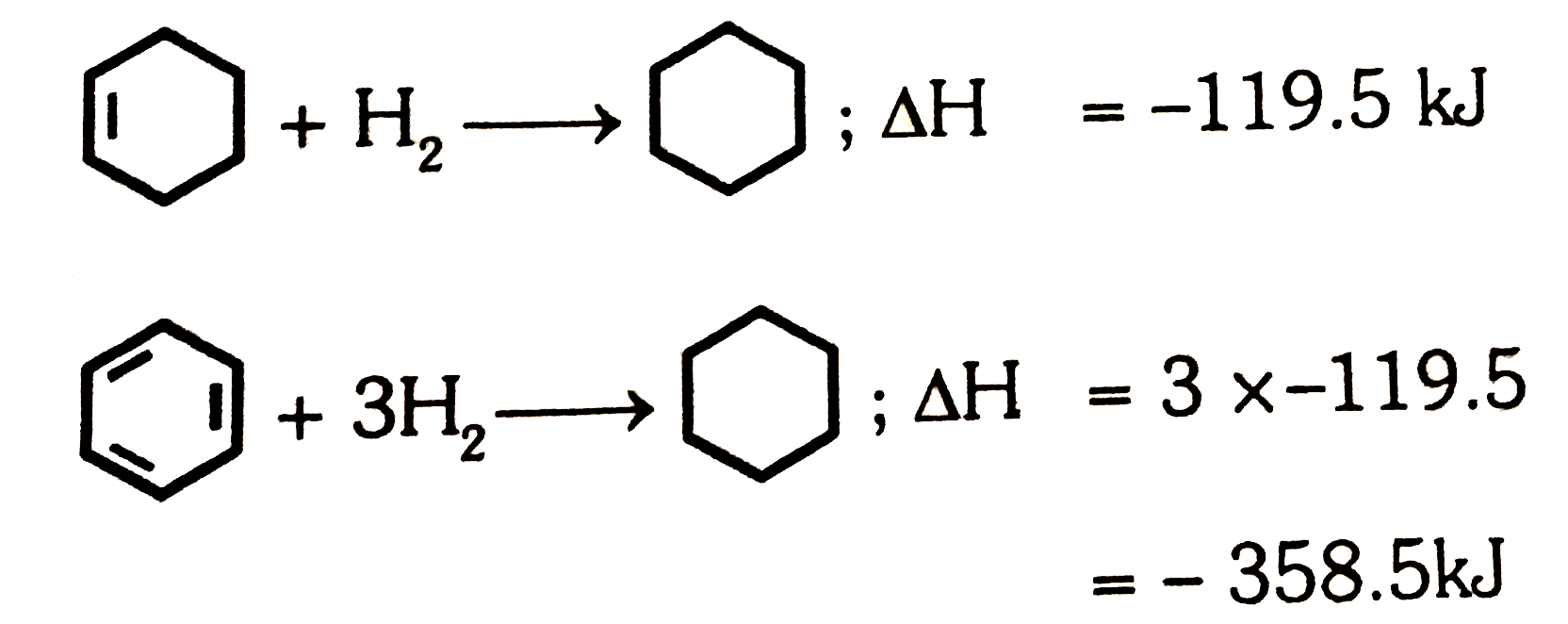A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-THERMODYNAMICS -EXERCISE -1A
- Assume each reaction is carried out in an open container. For which r...
Text Solution
|
- The enthalpy and entropy change for the reaction Br(2)(l)+Cl(2)(g)rarr...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexene is -119.5 kJ mol^(-1). If...
Text Solution
|
- For the reaction of one mole of zinc dust with one mole of sulphuric a...
Text Solution
|
- When you make ice cubes, the entropy of water
Text Solution
|
- For a phase change H(2)O(l) to H(2)O (g)
Text Solution
|
- For a spontaneous process the correct statement is
Text Solution
|
- The enthalpy change (Delta H) for the reaction, N(2)(g)+3H(2)(g)to 2NH...
Text Solution
|
- Consider the following reactions: (a) H(aq)^(+) +OH(aq)^(-) to H(2)O...
Text Solution
|
- Given that bond energies of H–H and Cl–Cl are 430 kJ mol^(-1) and 240...
Text Solution
|
- Bond dissociation enthalpy of H(2), Cl(2) and HCl are 434, 242 and 431...
Text Solution
|
- For the gas phase reaction, PCl(5)(g) hArr PCl(3)(g) + Cl(2)(g) Wh...
Text Solution
|
- Which of the following are not a constant value between two given ther...
Text Solution
|
- From the following bond energies: H-H bond energy: 431.37KJmol^(-1) ...
Text Solution
|
- The values of DeltaH and DeltaS for the reaction, C("graphite")+CO(2...
Text Solution
|
- For vaporization of water at 1 atmospheric pressure the values of Delt...
Text Solution
|
- Three moles of an ideal gas expanded spontaneously into vaccum. The wo...
Text Solution
|
- The following two reactions are known : Fe(2)O(3)(s) + 3 CO(g) rightar...
Text Solution
|
- Standard entropies of X(2), Y(2) and XY(3) are 60, 40 and 50 JK^(–1) m...
Text Solution
|
- Intensive property is :-
Text Solution
|