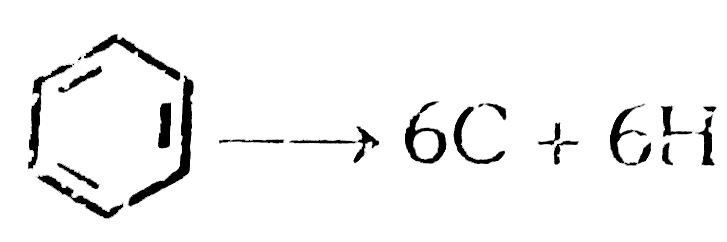A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-THERMODYNAMICS -EXERCISE -3
- The enthalpy changes at 298K in successive breaking of O-H bonds of ...
Text Solution
|
- If values of Delta(f)H^(Theta) of ICI(g),CI(g), and I(g) are, respecti...
Text Solution
|
- The heat of dissociation of benzene in isolated gaseous atoms is 5335 ...
Text Solution
|
- The enthalpy change for the reaction 2C("graphite")+3H(2)(g)rarrC(2)H(...
Text Solution
|
- Cl(2)(g)rarr2Cl(g), In this process value of Delta H will be -
Text Solution
|
- The magnitude of heat of solution ….. On addition of solvent to soluti...
Text Solution
|
- If H(2)(g) rarr 2H(g) " "DeltaH = 104 kcals Then heat of atomizat...
Text Solution
|
- The heat of combustion of yellow phoshphorus and red phosphorus are -9...
Text Solution
|
- For the change,C("diamond") rarr C("graphite"), Delta H = -1.89 kJ , i...
Text Solution
|
- 2CO((g))+O(2(g))rarr 2CO(2(g))+X KJ In the above equation X KJ refers ...
Text Solution
|
- Which of the following reactions represents Delta H (hydration) :-
Text Solution
|
- Delta H for the reaction, I((g))+I((g))rarr I(2(g)) will be :-
Text Solution
|
- Given that {:(A(s) rarr A(I)," ",Delta H = x),(A(I) rarr A(g)," ...
Text Solution
|
- The enthalpy change of a reaction does not depend on
Text Solution
|
- Form the thermochemical reactions. C(graphite) +1//2 O(2)(g) to CO(g...
Text Solution
|
- If H(2)+1//2O(2)rarr H(2)O , Delta =-68.39 Kcal K+H(2)O+ "water" rar...
Text Solution
|
- Given C(s)+O(2)(g)rarr CO(2)(g)+94.2 Kcal H(2)(g)+2O(2)(g)rarr CO(2)...
Text Solution
|
- From the following data, the heat of formation of Ca(OH)(2)(s) at 18^(...
Text Solution
|
- If H(2)(g) + Cl(2)(g) rarr 2HCl, Delta H^(@) = -44 kcal" "…..(i) ...
Text Solution
|
- In the reaction : S+3//2O(2) rarr SO(3)+"2x kcal" and SO(2) +1//2O(2) ...
Text Solution
|