A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-AIIMS 2019-CHEMISTRY
- Which of the following statements is true about compounds A-C below?
Text Solution
|
- Label each stereogenic center in the following compound as R or S.
Text Solution
|
- What is the correct IUPAC name of the compound?
Text Solution
|
- Which of the following compounds is expected to be optically active ?
Text Solution
|
- What is the stereo chemical relationship between the given compounds ?
Text Solution
|
- All the isomers with molecular formula C6H(12) that contain a cyclobut...
Text Solution
|
- The relationship among the following pairs of isomers is : I. A...
Text Solution
|
- The structural formula of sativene is shown below. How many stereogeni...
Text Solution
|
- Which of the following is true regarding the following conformations o...
Text Solution
|
- Number of Acyclic bromoalkene from the molecular formula C4H7Br (Struc...
Text Solution
|
- Total number of stereoisomers of the given molecule?
Text Solution
|
- (1) NH 4^(o+) overset (KOH) underset(K2HgI4)to Blue ppt (2) Ni^(...
Text Solution
|
- Which complex act as R.A.
Text Solution
|
- For the reaction : 2A + B to A2B , the rate = K[A][B]^2 with K = 2...
Text Solution
|
- Half life (t1) of the first order reaction and half life (t2) of the 2...
Text Solution
|
- Calculate the log(10) of the ratio of the catalysed and uncatalysed ra...
Text Solution
|
- The concentrations of the reactant A in the reaction A toB at differen...
Text Solution
|
- For an exothermic chemical process occurring in two as (i) A + B rar...
Text Solution
|
- A substance undergoes first order decomposition. The decomposition fol...
Text Solution
|
- The activation energy of exothermic reaction A rarr B" is 80 kJ mol"^(...
Text Solution
|
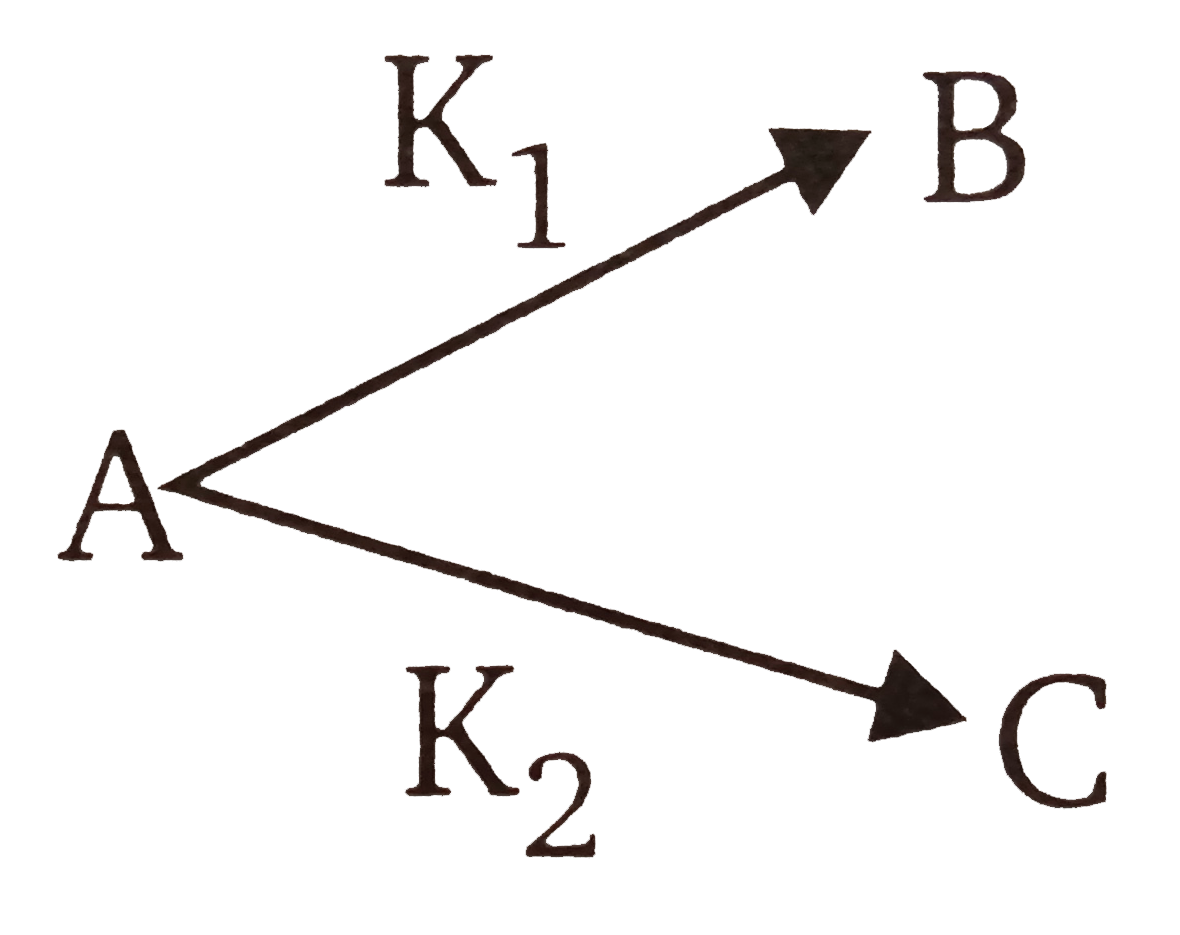 and `KP_(1)=1.26xx10^(-4)sec^(-1)`
and `KP_(1)=1.26xx10^(-4)sec^(-1)`