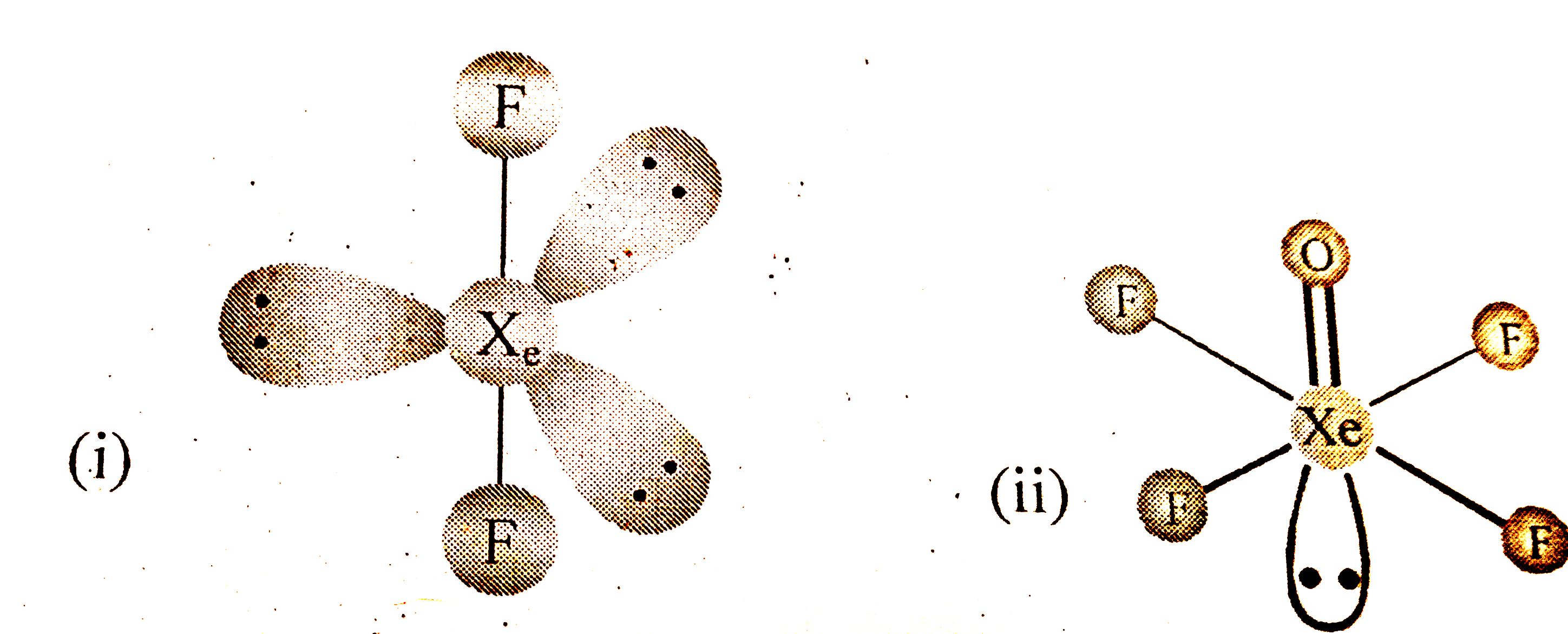Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-TEST PAPER 1-CHEMISTRY
- pK(a) value of 4-nitrobenzoic acid is lower than that of benzoic acid...
Text Solution
|
- Why is H(2)S a better reducing agent than H(2)O ?
Text Solution
|
- What happens when X eF(6) reacts with NaF ?
Text Solution
|
- Draw structures of the following derivatives. (i) The 2, 4-dinitro...
Text Solution
|
- Arrange the following compounds in increasing order of their reactivit...
Text Solution
|
- Draw structures of the following derivatives. (i) The ethylene ket...
Text Solution
|
- Write balanced chemical equations for the following processes : (a) ...
Text Solution
|
- Arrange the following compounds in the increasing order of their prope...
Text Solution
|
- Arrange the following in increasing order of their basic strength : ...
Text Solution
|
- (a) Fluorine is a stronger oxidizing agent than chlorine. Why ? (...
Text Solution
|
- (a) Account for the following : (i) Acidic character increases fro...
Text Solution
|