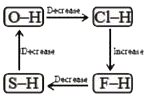
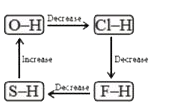
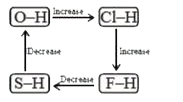
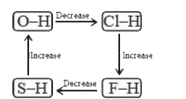
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-INORGANIC CHEMISTRY-METALLURGY
- Which of the following diagrams shows correct change in the polarity o...
Text Solution
|
- In order to refine "copper" it is melted in a furnace and it is stirre...
Text Solution
|
- The process which involves the treatment of the ore with a suitable re...
Text Solution
|
- Select correct matching :-
Text Solution
|
- Read the following statements :- (I) Al has greater affinity than t...
Text Solution
|
- Which of the following is incorrect ?
Text Solution
|
- Which of the reactions occurs in slag formation zone in blast furnace ...
Text Solution
|
- Giving suitable example, describe the importance of the formation of c...
Text Solution
|
- Both copper carbonate and copper hydroxide are present in :-
Text Solution
|
- Ag(2)S +underset("Excess")(KCN) to [Ag(CN)(x)]^(-n) [Ag (CN)(x)]^(-n...
Text Solution
|
- When ZnS and PbS minerals are present together, Then NaCN added to sep...
Text Solution
|
- Which of the following statement is not correct regarding calcination ...
Text Solution
|
- "Ore" overset("Calcination")to "Residue" overset("dil.HNO"(3))tounders...
Text Solution
|
- Which of the following is not correctly matched
Text Solution
|
- The method of zone refining of metals is based on the principle of
Text Solution
|
- Aluminium oxide is not reduced by chemical reactions due to
Text Solution
|
- Which of the following sulphide ore is not concentrated by froth-float...
Text Solution
|
- Which of the following statements is correct regarding the slag obtain...
Text Solution
|
- Which of the following factors is of no significance for roasting sul...
Text Solution
|
- In the context of the Hall-Heroult process for the extraction of Al, w...
Text Solution
|
- Which of the above curve is wrongly presented -
Text Solution
|