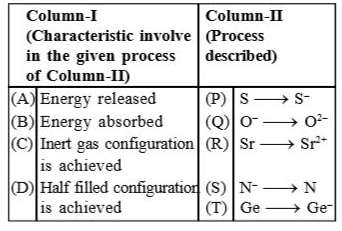
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
INORGANIC CHEMISTRY
ALLEN|Exercise CHEMICAL BONDING|119 VideosINORGANIC CHEMISTRY
ALLEN|Exercise COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS|109 VideosINORGANIC CHEMISTRY
ALLEN|Exercise METALLURGY|22 VideosHYDROGEN
ALLEN|Exercise EXERCISE-5|17 VideosIonic Equilibrium
ALLEN|Exercise All Questions|37 Videos