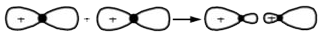
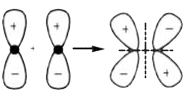
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
INORGANIC CHEMISTRY
ALLEN|Exercise COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS|109 VideosINORGANIC CHEMISTRY
ALLEN|Exercise COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS (MATCH THE COLUMN)|2 VideosINORGANIC CHEMISTRY
ALLEN|Exercise PERIODIC TABLE (MATCH THE COLUMN TYPE QUESTIONS)|5 VideosHYDROGEN
ALLEN|Exercise EXERCISE-5|17 VideosIonic Equilibrium
ALLEN|Exercise All Questions|37 Videos
Similar Questions
Explore conceptually related problems
ALLEN-INORGANIC CHEMISTRY-CHEMICAL BONDING
- The incorrect order of bond angle :-
Text Solution
|
- Which of following statement is incorrect.
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- Which of the following statements is not correct regarding bonding mol...
Text Solution
|
- Which of the following statement is correct : As the %s-character of a...
Text Solution
|
- Which of the following molecule has two lone pairs and bond angle lt 1...
Text Solution
|
- Which of the following does not contain PX(4)^(+) type cation in soli...
Text Solution
|
- Incorrect orders of bond angle is :
Text Solution
|
- In which of the following molecule 2pi and (1)/(2)sigma bond is presen...
Text Solution
|
- Order H(3)PO(4) gt H(2)SO(4) gt HNO(3) gt HCl is correct for :-
Text Solution
|
- In which of the following compound all the bond angles are equal :-
Text Solution
|
- Correct order of Bond angle is
Text Solution
|
- Which of the following do not exhibit resonance.
Text Solution
|
- The incorrect statement regarding chloral hydrate C Cl(3)CH(OH)(2) is ...
Text Solution
|
- Which of the following statements is incorrect
Text Solution
|
- Identify the correct option
Text Solution
|
- Hybridisation involves the mixing of orbitals having comparable energh...
Text Solution
|
- In which of the following molecules no. of lonepairs and bond pairs on...
Text Solution
|
- Number of carbon atom present linearly in C(3)O(2) :-
Text Solution
|
- In which pair both are not isostructural :-
Text Solution
|